- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7620 Courses
Elite EA PA Admin Forum 🇦🇺
By Elite Forums AU
Elite EA/PA Forum We are delighted to announce the Elite EA/PA Forum for ANZ will be on the 25th of September 2025. Our workshop aims to: Enhance your influence and impact by mastering assertive communication, negotiation, and self-advocacy skills. Stay composed and solution-focused in high-pressure situations with practical tools for crisis management and clear decision-making. Embrace evolving technology by understanding how AI and automation can streamline your role and boost efficiency. Future-proof your career by building strategic value, resilience, and adaptability in an ever-changing professional landscape. Keynote Address with Q&A from the audience: What to expect from Sam's Keynote: With over 30 years supporting leaders at the highest levels, Sam Cohen brings a wealth of insight, experience, and stories (the kind she can share) to the stage. From 18 years serving within The Royal Household - including as Deputy Private Secretary and Press Secretary to Her late Majesty Queen Elizabeth II - to working with The Duke and Duchess of Sussex, running the Prime Minister’s Office at Downing Street, and serving as Chief of Staff to the global CEO of Rio Tinto, Sam’s career is a masterclass in discretion, diplomacy, and delivering at the top. In this exclusive keynote, Beyond the Role, Sam will explore how Executive Assistants don’t just support leaders - they shape leadership, drive strategy, and build legacy from behind the scenes. Join us for this rare opportunity to hear from someone who’s been at the epicentre of power - and bring your questions! The keynote will include a live Q&A, where you can ask Sam about her remarkable career, leadership insights, and how to truly excel in high-performance environments. (Don’t ask her what the Queen kept in her handbag - she’s not telling.) Sam Cohen Career Bio: Sam Cohen has spent the last 30 years working to support leaders in the public and private sectors. Sam spent 18 years serving The Royal Household, as Deputy Private Secretary to Her late Majesty Queen Elizabeth II and Press Secretary to The Queen. Sam also served as Private Secretary to The Duke and Duchess of Sussex. Following this time, Sam worked as Director of the Prime Minister’s Office at Downing Street under Boris Johnson and, most recently, was Chief of Staff to the global CEO at Rio Tinto. Source: ABC News - YouTube Channel. Facilitator - AM: The Future-Proofed Assistant: Speak Up, Stand Out & Shape Your Career Path How to reimagine your Assistant role in 2025 and beyond - How the EA role is evolving (and what Executives now expect) & why Assistants who think like strategists will be indispensable Assertiveness as an Assistant – The key to retaining your role & the difference between being ‘helpful’ and being ‘heard’ Self-Advocacy as a Career Growth Strategy – The importance of advocating for your career development, workload boundaries and recognition Own Your Professional Future - Map your career development. Whether you're an EA for life or looking to a role beyond in the future, this is for you. Ruth Kilah Career Bio: Ruth is an international executive career coach and founder of Hoxton Hyde – Executive Career Coaching & Mentoring, delivering 1:1 and group programs for experienced Executive Assistants. She specialises in helping EAs step into higher-level roles, increase their income, and expand their professional impact. With 14 years’ experience supporting C-suite executives in Australia and the UK, Ruth brings deep industry insight and a strategic approach to career development. She empowers Assistants to gain clarity on their next move, adopt a strategic mindset, communicate their value effectively, and lead their own growth conversations with confidence. A former EA turned Stakeholder Relations and Project Manager, Ruth launched Hoxton Hyde in 2018 after spotting a clear gap in the market for tailored coaching for career-driven EAs. She is a respected thought leader in the EA space, regularly sharing insights via LinkedIn and Instagram. Panel: Crisis Mode: What to do when everything goes wrong Master a step-by-step approach to prioritising and problem-solving under pressure. Strengthen emotional resilience and calm decision-making during unexpected disruptions. Learn how to communicate clearly and lead from behind in high-stress situations. Emma-Kate Bos Bio Emma-Kate works alongside the CEO at Squadron Energy, one of Australia’s leading renewable energy companies. With over 28 years of experience in Executive Assistant and Operational roles supporting business leaders in professional services, politics, membership industries, sporting and not-for-profit organisations, Emma-Kate has a deep understanding of business support roles and has managed large teams of assistants and receptionists. She is passionate about developing and mentoring team members Emma-Kate holds an Associate Degree in Law, Mini MBA and Certificate in Public Relations. Sepi Nowlands Sepi has also worked as an EO for Deloitte and spent 18 years previously as an Executive Assistant at the ATO, Law Council of Australia, Grains Research and Development Corporation and Air Services Australia. She is now EA and EO to the CPO. Holly Clareburt Hollie Clareburt is an experienced Executive Assistant, currently supporting the Managing Director of Microsoft New Zealand and the Chief Partner Officer. Known for her professionalism and discretion, she excels in providing high-level support in fast-paced, executive environments. Prior to Microsoft, Hollie was Executive Assistant to the Chief of Corporate & Enterprise Systems at BECA, and previously supported the CEO at SKY News New Zealand. Her career reflects a strong track record of reliability, organisation, and executive partnership. Liv Wilson With over 20 years of experience across banking, government, creative industries, and global tech, Liv has supported senior leaders at companies including LinkedIn and Slack. She brings a strategic lens to the business support function, with deep expertise in operations, leadership enablement, and organisational effectiveness. As a passionate advocate for elevating the role of Executive Assistants, Liv has led women’s networks, championed DEI and social impact initiatives, and continues to push for recognition of business support as a critical driver of business success. She is currently working on her side hustle business - collaborating with executives, entrepreneurs, and small business owners to amplify their impact by removing operational barriers, optimising systems, and unlocking their capacity to lead and grow. Facilitator - PM: Justin Kabbani AI Is Not Here to Replace You. It's Here to Upgrade You. We'll explore Justin's proven 3P framework: Priming – How to set up AI like a strategic advisor by feeding it context, tone and mindset Prompting – How to craft clear, structured instructions to get consistently great results Producing – How to turn AI outputs into high-leverage work that makes you stand out Your session outcomes: Real examples from admin professionals already using AI to elevate their work Prompts you can copy, adapt, and test live Interactive exercises to build confidence fast A practical challenge to implement right after the session If you’ve been overwhelmed by AI, or underwhelmed by its impact, this session will change that. You'll leave with tools you can use today, and a mindset you’ll carry forward for the rest of your career. Justin Kabbani Career Bio: Justin Kabbani is one of Australia’s most in-demand AI trainers and keynote speakers, known for making AI feel simple, powerful, and immediately useful. He’s worked with brands like Uber, Treasury Wine Estates, and Udemy, helping their teams embed AI into daily workflows, strategic planning, and executive communication. Over the past two years, Justin has trained more than 2,000 professionals across Australia and beyond, consistently earning feedback like “mind-blowing,” and “game-changing”. His signature Prime, Prompt, Produce framework has transformed how business leaders, executive assistants, marketers, and teams think, work, and communicate with AI, without needing to be “tech people.” Justin believes AI isn’t here to replace people. It’s here to take the robotic work off our plate, so we can focus on what humans do best. LinkedIn: https://www.linkedin.com/in/justinkabbani/ Website: https://justinkabbani.com/ Speed Connections Networking Session Join us for Speed Connections, a lively 30-minute networking session designed to foster meaningful connections in a fun, fast-paced environment. Every 10 minutes, attendees will be placed into new breakout rooms with small groups, giving everyone the chance to meet a diverse range of peers. Each breakout session will feature engaging prompts to spark conversations and make networking enjoyable and memorable! Who will attend this event? Executive Assistant (EA) Personal Assistant (PA) Virtual Assistant (VA) Legal Secretary Legal Executive Assistant Administrative Assistant Office Manager Health Care Office Manager Chief of Staff Additional roles may be relevant depending on role responsibilities along with development opportunities. We understand the challenge. Professional development for assistants is often undervalued, and securing budget or approval for external training can be difficult. That’s why we’ve created a ready-to-use business case template to help you justify attendance at this event and highlight the value it brings to both you and your organisation. If you need support or costings confirmed for single or group attendance, please reach out to our support team at: support@elite-forums.com You can download and edit the template below: This workshop is open to females, male including trans women/males and non-binary professionals. Group Rate Discounts: To discuss our group rates in more detail, please email support@elite-forums.com and provide the following: Group Number (How many would like to attend) Event Date (If numerous dates, please advise if we are splitting attendees across multiple dates) Attendee Contact details (Or request our Group Rate Document. Complete and return - we'll sort the rest.) Group discounts are on request - see below group rate discount brackets: 🧩 You just need one piece to come together - to unlock your Elite Potential. 🔑 Please note: All facilatators, panelists, and speakers are all PAID with applicable contracts in place. We value our speakers and want the best to ensure our attendees get the best development. Media outlets/organisations will not be permitted to attend this event.

3-Day First Aid at Work Course
By Madeleys First Aid Plus
🩺 Be Prepared. Be Confident. Be a Lifesaver. 🩹 Is your workplace ready for emergencies? Our 3-day First Aid at Work qualification meets the legal requirements under the Health and Safety (First Aid) Regulations 1981 and (Northern Ireland) 1982. ✅ Gain essential knowledge ✅ Build practical first aid skills ✅ Be ready to respond in a wide range of workplace situations Perfect for: 🏢 Workplaces with identified first aid needs 🤝 Voluntary and community groups 🧑🤝🧑 Anyone responsible for first aid provision 📅 Don’t wait for an emergency to happen—get trained and qualified today! #FirstAid #WorkplaceSafety #HealthAndSafety #FirstAidTraining #BePrepared #LifesavingSkills

Principles of Safeguarding and Protecting Children, Young People or Vulnerable Adults
By Madeleys First Aid Plus
🛡️ Protecting the Most Vulnerable Starts with You 🧒👵 The RQF Level 3 Safeguarding course empowers you with the knowledge to recognise, respond to, and report signs of abuse or neglect. 📘 Learn how to: 🔍 Identify signs of abuse 📞 Report concerns appropriately 🛠️ Take action to ensure safety and well-being 👥 Understand your responsibilities in safeguarding children, young people, and vulnerable adults Whether you work in education, healthcare, social care, or community services — this course is essential for creating safe and supportive environments. 📅 Enrol today and be a voice for those who need it most. #Safeguarding #ProtectVulnerablePeople #ChildProtection #SafeguardingTraining #RQFLevel3 #DutyOfCare #MakeADifference

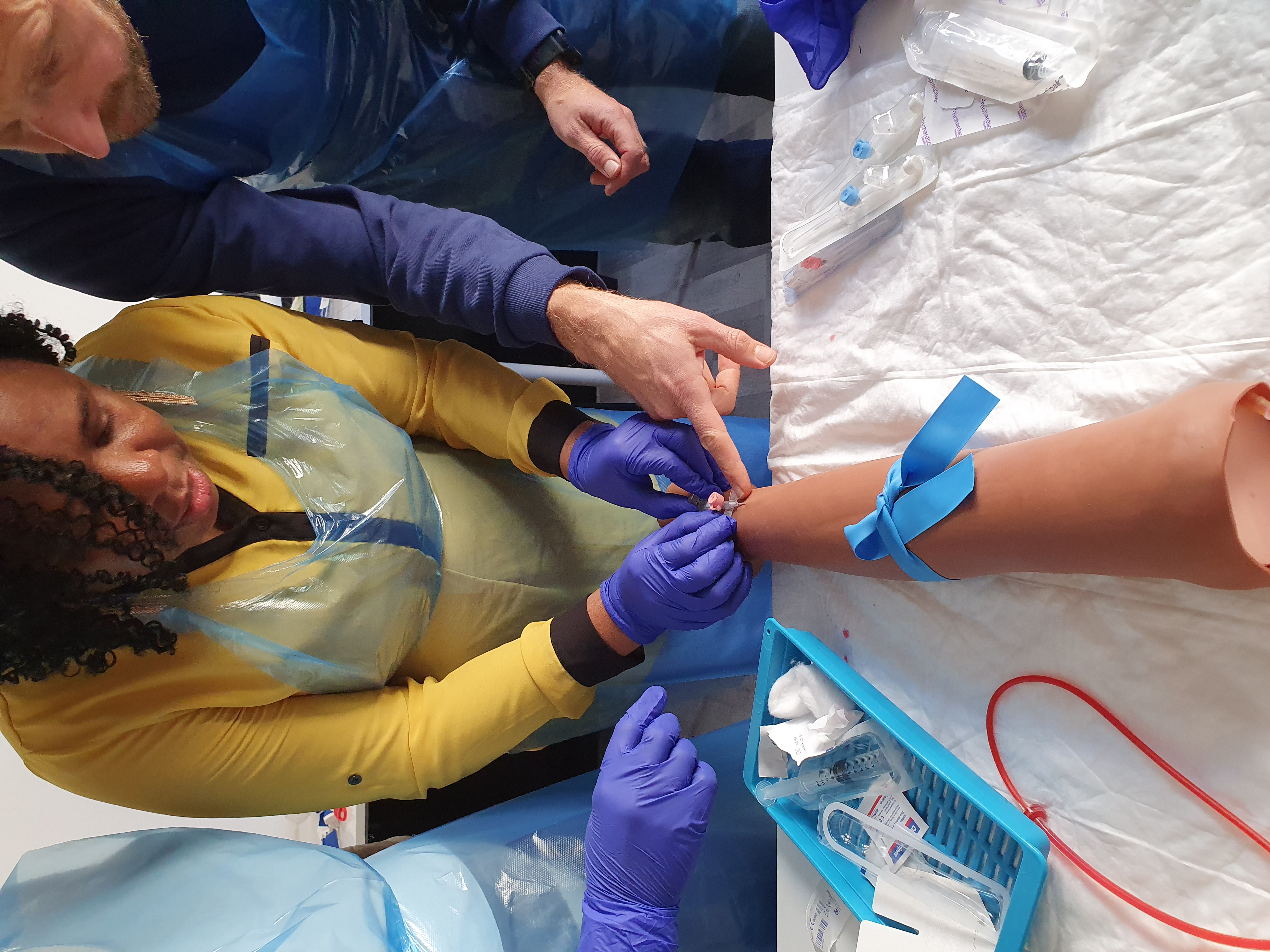
Venepuncture and cannulation course Venepuncture training for healthcare professionals Cannulation skills development Intravenous access techniques Blood sample collection training Infection control in venepuncture Hands-on venepuncture practice Cannula insertion training Nursing revalidation hours CPD accredited course Healthcare professional skills development Venepuncture and cannulation procedures Venepuncture certification program Intravenous catheter insertion Best practices in venepuncture Patient assessment for venepuncture Troubleshooting venepuncture complications Venepuncture and cannulation simulation Real-life venepuncture scenarios Healthcare career advancement with venepuncture skills
This programme helps communicators to prepare for and deliver an effective speech. Here, you learn to motivate people by speaking to them in terms of the benefits they will receive by taking action. Each instructional segment is followed by participant speeches that put the newly-learned skills into action. We cover public speaking fear and how to move forward despite it, as well as increasing enthusiasm. This builds speaker confidence. By organising information clearly for the audience, participants grow in their conviction and are perceived more as experts. Delegates will be able to: Work through fear of public speaking; Build confidence and enthusiasm by creating meaningful, memorable speeches; Develop greater abilities for thinking and speaking with less preparation; Minimise self-defeating speech and behavior; and Present ideas to, and inspire the audience. Online Format—Introduction to Public Speaking is a 4-hour interactive virtual class. Register for this class and you will be sent ONLINE login instructions prior to the class date. Working with Dr. Atkins of Improving Communications has been a very positive experience. Everything about the program is exemplary! Managers have made it a point to tell me how pleased they are with the improvements made in the communications skills of the participants. The Professional Development Workshop is an extremely effective program. The participants are looking forward to follow-up sessions with Dr. Atkins and we recommend his programs. Heather Ragone, Training ManagerNetwork General

, The Intravenous Route, Bioavailability, the First Pass Effect, IV drug administration Vascular Access Devices Care & Management: Peripheral Cannula, Midline, Catheter, different Central Venous Access(care of Hickman line), PICC, Implantable Port, UVC and subcutaneous infusion, VAD Assessment, Risk & Complication of IV Therapy, Infection control, Allergy, Fluids & Electrolytes and Drug Calculations. Total Parenteral Nutrition –TP, Solution content, Administration, Routes for delivery,
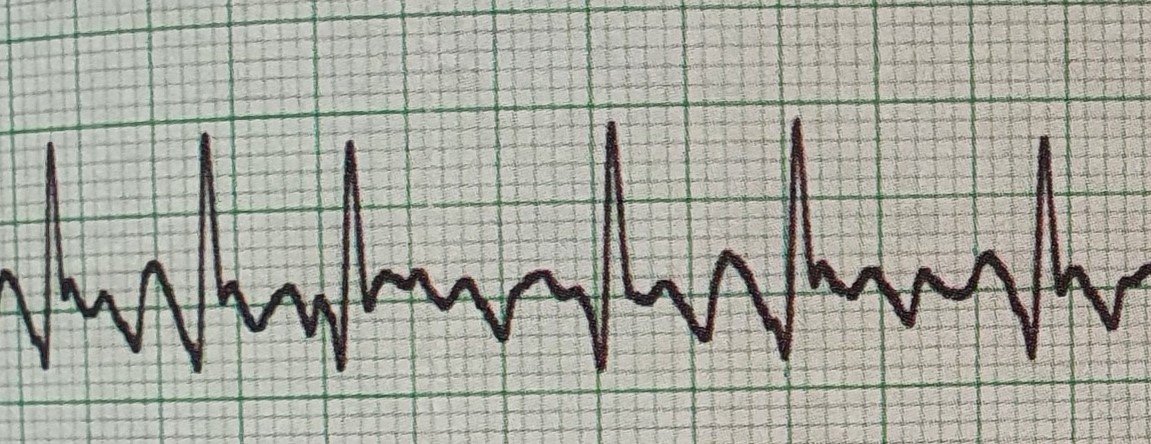
Basic ECG interpretation ECG basics for beginners ECG course for healthcare professionals ECG training for nurses Beginner ECG reading skills Introduction to ECG interpretation Understanding ECG rhythms Identifying common ECG abnormalities ECG strip reading practice ECG lead placement ECG graph paper essentials Interpreting normal sinus rhythms Recognizing cardiac arrhythmias Practical ECG exercises Hands-on ECG interpretation Expert instructors in ECG training CPD accredited ECG course 7 hours toward nursing revalidation Healthcare professional ECG certification Real-world ECG scenarios
Awareness of Safeguarding
By Madeleys First Aid Plus
Course Description The RQF Level 1 Awareness of Safeguarding course is designed to provide individuals with a basic understanding of safeguarding principles and practices. It aims to raise awareness about the importance of safeguarding and promote the well-being and protection of vulnerable individuals, such as children, young people, and adults at risk. The course covers the following topics: Introduction to Safeguarding: Definition and importance of safeguarding. Key legislation, policies, and guidance related to safeguarding. Roles and responsibilities of individuals and organisations in safeguarding. Types of Abuse and Neglect: Overview of different types of abuse, including physical, emotional, sexual, and financial abuse. Recognizing signs and indicators of abuse and neglect. Understanding the impact of abuse on individuals' well-being. Vulnerable Groups: Identifying vulnerable groups, such as children, young people, older adults, and individuals with disabilities or mental health issues. Understanding the specific safeguarding concerns and considerations for each group. Reporting and Responding to Safeguarding Concerns: Procedures for reporting safeguarding concerns or disclosures. Understanding the importance of maintaining confidentiality and handling sensitive information appropriately. Responding to safeguarding concerns in a timely and appropriate manner. Promoting Safeguarding and Preventing Abuse: Strategies for promoting a safe and inclusive environment. Recognizing potential risk factors and implementing preventative measures. Understanding the importance of creating a culture of safeguarding within organizations. Multi-Agency Collaboration: Collaboration between different agencies and organisations involved in safeguarding, such as social services, law enforcement, and healthcare. Sharing information and working together to ensure effective safeguarding practices. Case Studies and Scenarios: Reviewing case studies and scenarios to apply safeguarding principles and practices. Analysing potential safeguarding dilemmas and decision-making processes. Personal Responsibilities: Recognising personal boundaries and limitations when working with vulnerable individuals. Understanding the importance of self-care and managing emotional well-being when dealing with safeguarding issues. It is important to ensure that the course meets local safeguarding guidelines and requirements.

Search By Location
- Professional Development Course for Educators Courses in London
- Professional Development Course for Educators Courses in Birmingham
- Professional Development Course for Educators Courses in Glasgow
- Professional Development Course for Educators Courses in Liverpool
- Professional Development Course for Educators Courses in Bristol
- Professional Development Course for Educators Courses in Manchester
- Professional Development Course for Educators Courses in Sheffield
- Professional Development Course for Educators Courses in Leeds
- Professional Development Course for Educators Courses in Edinburgh
- Professional Development Course for Educators Courses in Leicester
- Professional Development Course for Educators Courses in Coventry
- Professional Development Course for Educators Courses in Bradford
- Professional Development Course for Educators Courses in Cardiff
- Professional Development Course for Educators Courses in Belfast
- Professional Development Course for Educators Courses in Nottingham

