- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
65618 Courses
Technical Author/Technical Writing Course (Independent study option)
By Cherryleaf
Efficient ways to create professional-looking diagrams, images and screenshots Cherryleaf’s popular, and affordable, online Technical Author/technical writing course teaches you the key skills of technical writing for IT and software user documentation. It's ideal if you’re: Looking to start your career as a Technical Author, Technical Writer, or similar role Writing instructions, but you’ve never had any proper training in how to do this well You’ll gain confidence you’re approaching each writing project in the right way, and creating content that follows best practice. This technical communication course is accredited by the Institute of Scientific and Technical Communicators The Technical Author/technical writing training course’s content is accredited by the Institute of Scientific and Technical Communicators, the professional body for Technical Communicators in the United Kingdom. It is an independent assessment of the course’s quality. This course is also recommended by the Society for Technical Communication (USA), the Australian Society for Technical Communication, and the Technical Communicators Association of New Zealand to their members. Learn from the experts As well as teaching technical communication, we also create end-user documentation, Help and UI text for clients. This means every course is based on practical experience of technical writing in today’s environment. The techniques you’ll learn on this course are the same ones used by our projects team. Professional certification as a technical communicator Cherryleaf’s training courses can help you be admitted to the national professional body for technical communicators in the UK (it also has members around the world) - The Institute of Scientific and Technical Communicators (ISTC). Junior Members, Members and Fellows are certified by the ISTC through a vetting process, with Members and Fellows entitled to use the initials MISTC or FISTC after their names. Being a member of the ISTC can help in having your skills and experience recognized by others. Comments from delegates There is something new to learn for most technical authors who are in the early stages of their career. Overall, I would say that the course did benefit me, and I can see myself revisiting parts of it in the future. Emma W., ARM plc I attended a job interview which included a written exercise where I had to jot down how I would go about designing and presenting information for the users of the service unit I was applying to. I could not believe my luck, it was as if the interviewer had designed it with the contents of this course in mind, and, as a result, I got the job! Amber A. I thought it was an excellent course – very thought-provoking and useful. The exercises were enjoyable and really helped, far beyond simply reading from a book about the tasks involved in the job. And I greatly appreciate all your advice and support. Karl D. Thank you very much for all your feed-back – I’ve been reading through this as it has come in this morning. This is really useful and very much appreciated. David H. Who is this course for? This course is ideal for you if you need to: Create end-user documentation and other forms of instructional content with ease Master the fundamentals of technical writing Get a solid understanding of the Technical Author’s role. This course is ideal for more experienced technical writers who’ve never had any formal training. What is a Technical Author? Technical Authors are sometimes known as: Technical Communicators, Technical Writers, Content Developers, Information Developers and Documentarians. Based in the United States or mainland Europe? We've designed the course to work for people living all around the world. On the videos, you'll hear a lovely, clear British accent. What will I learn? In this course, we’ll take you through the process of creating technical documentation. You’ll learn and practise: The purpose and value of technical documentation (aka having an answer if people ask why you should be doing this work). The entire documentation process. We’ll explain what you need to do, from beginning to end. We cover planning, writing, editing, indexing, and production. The skills you need as a technical communicator. How to work more efficiently. This framework will help you avoid wasting time. How to work more effectively. We’ll show you how to write and design your information in a structured, useful, usable and informed way. We don’t like to overhype our courses, so here are some reasons why not to get our technical writing training course. Those are: If you have requirements for your documents to meet specific regulatory documentation standards in these industry sectors: aerospace, military, railway, or automotive. Where documentation must be written to a specific XML standard. (You’ll need to take our DITA module in our advanced course as well). If you need to write technical API documentation for developers. (You’ll need to take our API module in our advanced course as well). Apart from that, this course will help you create the user assistance for your application or product. Duration and delivery format The course comprises 15 modules in total, which you can complete at your own pace. The course modules are delivered over the Web in small, manageable video presentations. You can download the course handouts as Word or PDF files. With the time needed to complete the exercises, you will need to allocate around two days in order to complete the course. You can pause the videos at any time and return to the course at times that suit you. Your Instructor Cherryleaf Cherryleaf is a technical writing services company formed in 2002 by people with a passion for technical communication and learning development. Cherryleaf is recognised as a leader within the technical communication profession. Our staff have written articles for the Society for Technical Communication's (STC) Intercom magazine, the Institute of Scientific and Technical Communicator's Communicator journal and tekom's TCWorld magazine. They've also written books on technical communication. We've presented webinars for Adobe, Madcap Software or the STC, and we've spoken at various conferences around the world. Today, organisations throughout Europe use Cherryleaf’s services so they can provide clear information that enables users and staff to complete tasks productively. Course Curriculum First Section The purpose of technical documentation (7:17) The different models for technical documentation (9:52) What is a technical author? (21:24) Adopting a user-centred design approach (10:38) Overview of the writing process (10:40) Planning your content (10:24) Researching the product and audience (13:20) Software tools (10:40) Writing the topics – Overview (30:31) Writing the topics – How to write and present different types of information (13:33) Writing the topics – Using images and videos (11:43) Writing the topics – Index, Search and Metadata (16:57) Hyperlinking (5:20) Reviewing and editing (9:42) End of course (7:21) Bonus content Frequently Asked Questions When does the course start and finish? It is a completely self-paced online course - you decide when you start and when you finish.How long do I have access to the course?You have access for 12 months.What if I am unhappy with the course?We would never want you to be unhappy! If you are unsatisfied with your purchase, contact us in the first 30 days and we will give you a full refund.

This offering is for an initial session exploring your enneagram map report , which is included. The test I use is from Aephoria, and it will give you your tritype (your strategies for thinking, feeling and doing) as well as your instinctual variant, which is helpful information about where you tend to focus your attention. Once you have your map, we will unpack the information together, and if you wish, go on to explore it over a series of sessions, using creative tools and practices to find its meaning and wisdom for you in your life.

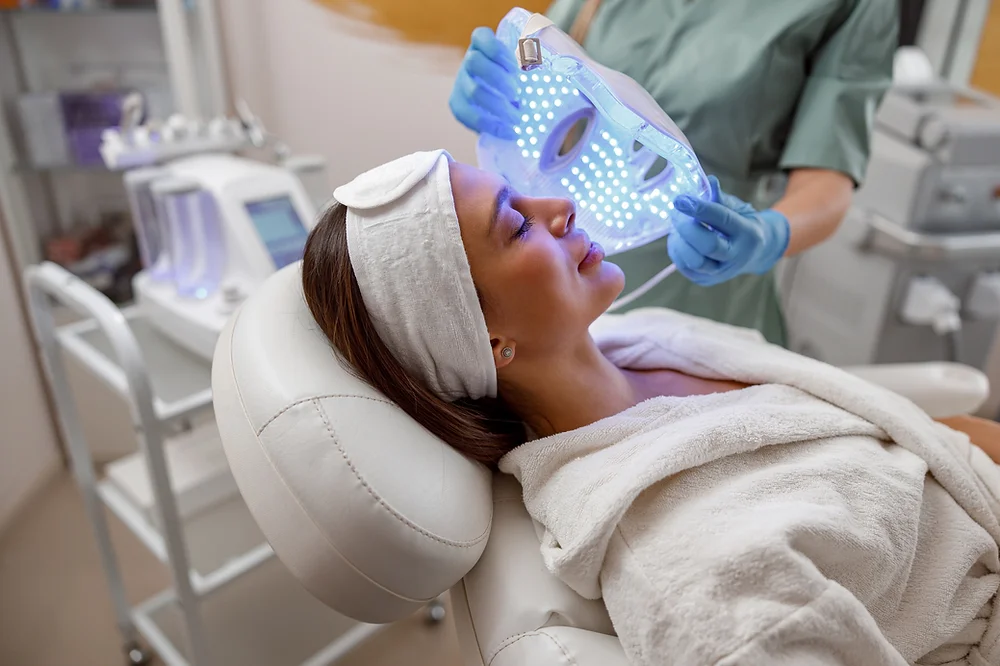
LED Light Therapy Course in person
By KBH Training Academy
LED Light Therapy Course What is LED Therapy? LED light therapy are a popular non-invasive skin treatment for acne, sun damage, wounds, and other skin problems Course content * Health and safety * Sterilisation and disinfection * Appearance of the therapist * Ergonomics * Handwashing * What is LED light therapy * Skin ageing and turning back time * Free radicals, radicals and anti-oxidants * How does LEAD work * The consultation process * Key points of LED * Benefits of LED * The treatment explained * Other benefits to LED therapy * The wound healing process * Important reminders and treatment tips * Treatment protocols Training kit if purchased -Facial kit -cleanser -Led bridge -Sponges 2x How does the course work? The course is divided into 2 parts, the first part is theoretical which you have to complete before you come for your practical training, and the second one is a practical assignment. The practical assignment is done on the day which will be agreed upon course purchase. You will spend around 2-3 hours practising on a model in our venue in London E106RA. Will I require a model? Yes, usually 1 model is required Do I Need Experience Before Booking a Course? We’re pleased to offer courses to people with lots of different experiences. However, previous experience nor qualifications are not necessary if you would like to enrol on our Course. Certificate You will receive an end of course certificate which is accredited by the cpd group and allows you to work on public Payment By paying for the course you agree to our terms and conditions.
1 Day beginners Fused Glass Wall Panel - Vessels class
By The Silver Zebra Glass Studio
Fused glass courses & workshops in the heart of Cheshire. Create your own unique fused glass wall panels, they can be split into 2 panels or work on 2 vessels if you prefer.

5 week interior design course
By SKELA STUDIO
Unlock your potential and embark on an inspiring journey in interior design. Whether you’re an aspiring professional or simply a design enthusiast, our workshops offer a safe and supportive space for you to flourish. Let’s make your design dreams a reality together. Embark on a thrilling expedition into the realm of Interior Design with us. Since 2016, we’ve been conducting captivating Interior Design workshops and courses in Edinburgh, witnessing a multitude of skilled individuals relishing the experience of acquiring new proficiencies. Our offerings are well-suited for both complete novices and those seeking to rejuvenate their expertise. Whether your aspiration is to revamp a room in your abode or simply take a tentative step into the universe of interior design, our workshops and courses are meticulously designed to empower you with the skills and assurance needed to master any interior design venture. What Will You Learn? In our workshops, you’ll master various skills, including: Gaining the confidence to design small interior spaces Creating compelling room plans and sections Skillfully using colour in interiors to set the right mood Enhancing your drawing skills to bring ideas to life Effectively incorporating texture and pattern into your designs Utilising lighting to craft captivating atmospheres in your spaces Attendees can request refunds up to 30 days before the event’s start date. Duration: 5 weeks Timings: TUESDAYS, 6 pm – 7 pm BST Location: ONLINE (ZOOM LIVE TALKS)

This offering is for an initial session exploring your enneagram map report , which is included. The test I use is from Aephoria, and it will give you your tritype (your strategies for thinking, feeling and doing) as well as your instinctual variant, which is helpful information about where you tend to focus your attention. Once you have your map, we will unpack the information together, and if you wish, go on to explore it over a series of sessions, using creative tools and practices to find its meaning and wisdom for you in your life.

Advanced Care Assistant & Care Planning- QLS Endorsed
By Imperial Academy
The UK is in dire need of care workers. One report suggests that 8.2% of care roles are unfilled. Up for a challenge?

Brain and Behaviour in Organisations
By Ideas for Leaders
Image Caption Your Master Mentor: Prof Paul Brown Applied neuroscientist, Prof Paul Brown introduces his General Theory on Individuals and Organisations in this ground-breaking program on how to create a 21st century sustainable organisation. Prof Paul Brown briefly introduces the 'Brain and Behaviour in Organisations': Applied neuroscience is going to be the need-to-know basis for anyone interested in human behaviour in organisations. Understanding how the brain is the master controller of everything we do, the role our emotions play in that and the Limbic Leadership qualities we need to adopt to self-regulate ourselves and tune in to those of our team. Course Content In this eight-part program, Prof Brown introduces you to how the brain is actually the central controller of everything we do. And how our increasing knowledge of the neurosciences can help us mobilise that knowledge in organisations. In the program Paul will introduce you to the idea that it is not our Thinking system that drives everything we do but our Emotions. We want to develop an understanding of how to create the relationships where people bring their best to the organisation. We're introduce the London Protocol of the Emotions, a working model of how it all happens. And discuss the differences and benefits of male and female energy. How can you lead and mobilise the best in both men and women in organisations in order to actually get the diversity that male and female brains bring. How can we manage stress and pressure better in the 21st Century Sustainable Organisation. The program looks at the Yerkes -Dodson law which tells us a great deal about how stress impairs performance. But most of all, we want to fascinate and excite interest in you about this remarkable organ that at last, we're beginning to understand join us if you can.
Cosmic Christ Series: Online Courses
By Katarina Heuser
Discover your unique Ascension skills and powers, receive insights and deep healing together with an abundance of light codes taking your life to the next level!

Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham
