- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
14484 Courses
Getting Started with Freelancing - A Practical Guide for Web Developers
By Packt
Learn actionable steps to launch a successful career as a freelance web developer with the help of an experienced professional

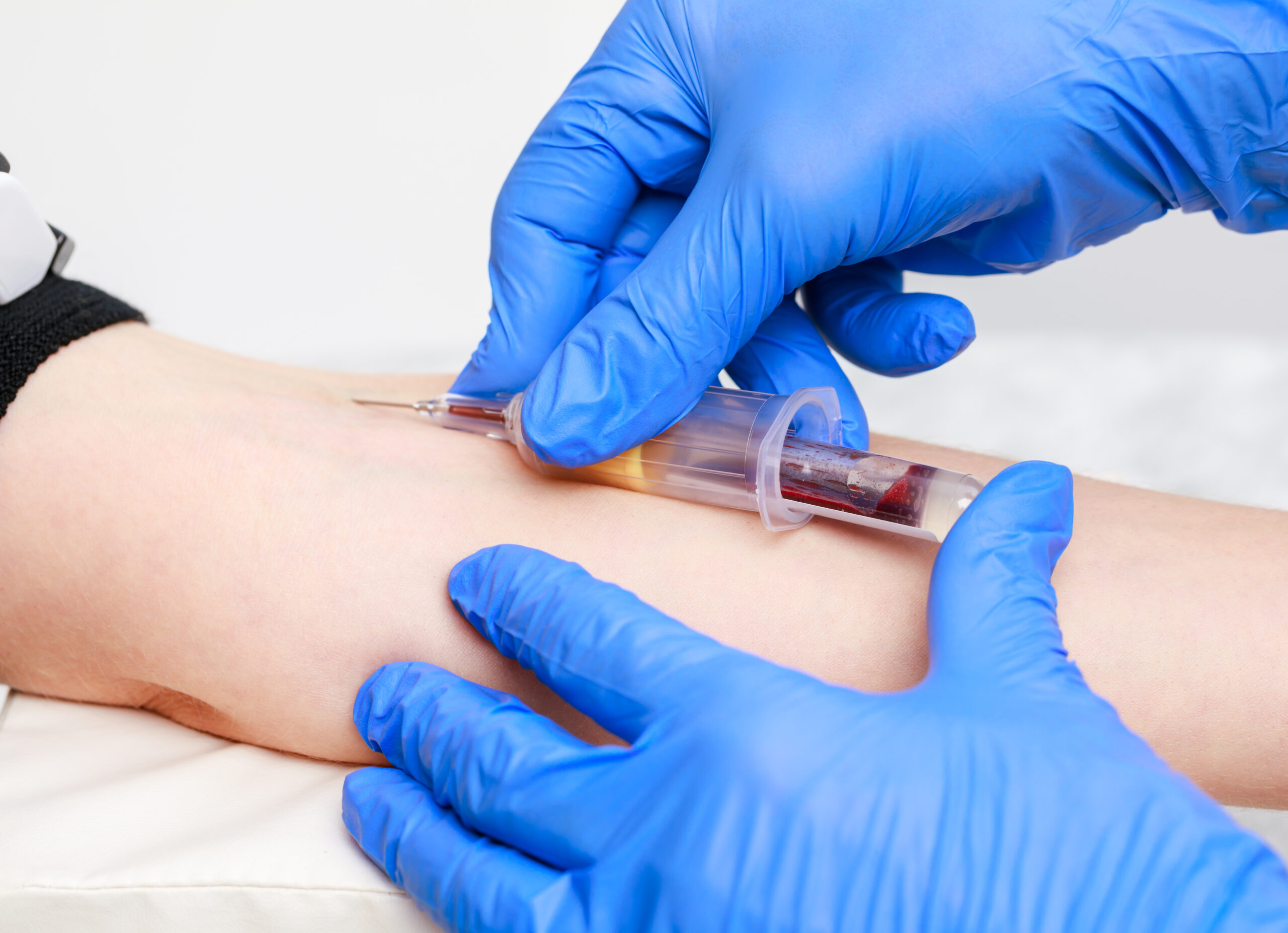
Promoting Best Practice in Venepuncture Instruction
By Guardian Angels Training
Enhance your venepuncture skills with our comprehensive course. Learn evidence-based techniques, infection prevention measures, patient-centered communication, and ethical considerations to ensure safe and effective practices.
Overview SEO help you define your most profitable focused audience and develop a bridge of communication that reach them directly, thereby positioning your product in the right and most efficient way. Using SEO skills targets increasing the number of visitors to a website and ensuring that the site appears high in ranking on the list of top page results returned by a search engine. SEO has become an increasingly sought-after skill for companies who want to keep track of their rankings in Google's organic search. To reach the customer nationally or internationally, SEO is the only way where we can reach a global market. It is very important to understand the techniques of SEO and how to use Digital Marketing in a more efficient way to reach every corner of the world. This Specialized course will highlight the skills required to optimize website content for top SE Ranking. Through this course, you will learn the algorithms many search engines follow including Google Search. Specially designed with a lot of practical and live examples of advanced SEO techniques used by professionals you will gain a real-world skill to enhance your knowledge in Content Marketing, On-Off Page Optimization, followed by aligning SEO with overall business strategies.

Developing Leadership and Emotional Intelligence Skills course is designed to empower participants with the essential leadership competencies and emotional intelligence skills needed to excel in today's dynamic workplace. Through a blend of theoretical frameworks, interactive exercises, and practical applications, this course aims to enhance participants' self-awareness, interpersonal relationships, and leadership capabilities, ultimately enabling them to inspire and motivate others towards achieving organizational goals.

Interview Mastery: Practical Strategies for Professional Success
By Compete High
ð Unlock Your Career Success with 'Interview Mastery: Practical Strategies for Professional Success' Course! ð Are you ready to transform your interview skills and land that dream job? Welcome to the ultimate guide for mastering interviews and securing professional success! This comprehensive online course is meticulously crafted across 13 modules to equip you with the knowledge and strategies needed to ace any interview scenario. Module 1: Interview Masterclass - Max Your Chances ð¯ Learn the crucial elements that maximize your chances of success in any interview setting. Module 2: Staying Professional ð Discover the art of maintaining professionalism throughout the interview process, leaving a lasting positive impression. Module 3: That Wasn't What You Expected ð¤ Navigate unexpected interview situations with grace and confidence, turning surprises into opportunities. Module 4: Waiting For The Answer â³ Understand the post-interview waiting period and strategies to handle this crucial phase effectively. Module 5: Let's Review The Tips - So Much To Consider ð Review and reinforce essential tips to consider before stepping into the interview room. Module 6: Get Yourself Ready! Power Preparation ð Dive deep into powerful preparation techniques that set you apart from other candidates. Module 7: Final Prep Tips! 1-2 Days Before Interview ð Fine-tune your preparations in the final days leading up to your interview for peak performance. Module 8: It's Interview Day! How to ACE the interview! ⨠Master the art of acing your interview day with confidence and poise. Module 9: Positive Personality Traits ð Discover and leverage positive personality traits that leave a lasting impact on your interviewers. Module 10: Conversational Gotchas ð£ï¸ Uncover potential conversational pitfalls and learn how to navigate them effortlessly. Module 11: Asking Questions ð¤ Master the skill of asking insightful and impactful questions during interviews. Module 12: Answering Questions ð¡ Learn effective techniques to confidently and eloquently answer any interview question thrown your way. Module 13: After Interview - Still More You Can Do ð Explore post-interview strategies to leave a lasting impression and follow up effectively. Enroll now and equip yourself with the tools and techniques necessary to excel in any interview scenario! Gain access to expert advice, practical tips, and actionable strategies that will propel your professional success. Don't let another opportunity slip away-start your journey to interview mastery today and unlock the career of your dreams! ðâ¨ð Course Curriculum Job Interview Practical's - Complete Interview Skill Training Interview Masterclass - Max Your Chances 00:00 Staying Professional 00:00 That Wasn't What You Expected 00:00 Waiting For The Answer 00:00 Let's Review The Tips - So Much To Consider 00:00 Get Yourself Ready! Power Preparation 00:00 Final Prep Tips! 1-2 Days Before Interview 00:00 It's Interview Day! How to ACE the interview! 00:00 Positive Personality Traits 00:00 Conversational Gotchas 00:00 Asking Questions 00:00 Answering Questions 00:00 After Interview - Still More You Can Do 00:00

Certified Data Protection Officer Training (CDPO)
By Training Centre
The IECB Certified Data Protection Officer training course will help you acquire the knowledge and skills to serve as a Data Protection Officer (DPO), allowing you to help organizations understand and implement solutions that meet the compliance requirements of the General Data Protection Regulation (GDPR), as well as a number of other regulatory requirements. The course takes a practical look at the GDPR requirements and advises on the mapping of solutions. In this way, delegates can master the role of the DPO and become competent to inform, advise, and monitor compliance with the GDPR and cooperate with the supervisory authority. About This Course After attending the training course, you can sit for the exam, and gain the 'Certified Data Protection Officer' credential, which validates that you have the Legal and regulatory knowledge required of a key adviser and practical knowledge to advise organisations how to meet their obligations regarding the GDPR compliance Learning Outcomes; Acquire a thorough understanding of the basic concepts and components of global Data Protection Regulation(s) Understand the correlation between the General Data Protection Regulation and best practice standards such as ISO 27701 Acquire a thorough understanding of the data protection by design requirements, particularly in relation to the protection of data Interpret the data protection requirements within the context of an organization Understand how to support an organization to plan, implement, manage, monitor and maintain ongoing compliance to the GDPR Our approach This training course is based on the practical application of best practices used in exercising the role of the DPO. Course modules include practical examples of the role of DPO. Delegates are encouraged to engage in discussions and exercises. A Case Study which brings the materials to life. Course agenda Day 1: Introduction to the GDPR Principles Day 2: The role of the DPO in the determination of a GDPR compliance program Day 3: The role of the DPO in business operations Day 4: Monitoring and maintenance of the GDPR compliance programme, as well as the examination Prerequisites A basic understanding of the GDPR will benefit course delegates. What's Included? Refreshments & Lunch (Classroom only) Course Slide Deck Official Study Materials CPD Certificate The Exam fees Who Should Attend? Individuals seeking to move into the role of DPO Team members who define, implement, and maintain a GDPR compliance programme Information Security Managers responsible for the personal data protection of an enterprise and the management of its risks Members of an information security, risk management, or Data Governance team Our Guarantee We are an approved IECB Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and train for free, with the exam retake included too. Assessment All candidates at official training courses will be offered the opportunity to sit the associated exam. For CDPO, this constitutes a 12 question essay type exam which should be completed within 240 minutes. A passing score is achieved at 70%. Accreditation Provided by This course is Accredited by NACS and Administered by the IECB.

February 2025 - 1 Day Practical Heat Pump Surveying Course (5 CPD Hours)
By Building Metrix
This Renewables Technical Surveying training short two day course is specifically designed for individuals and companies that want to train themselves and their staff on exactly how to carry out Renewables Technical Site Surveying prior to any renewables installation measures, this includes for heating systems, solar systems and EV Charge point installations. The course is primarily aimed at Energy Suppliers, Equipment Manufacturers, Renewable Installers, Domestic Energy Assessors, Retrofit Assessors, Retrofit Co-ordinators, Renewables sales staff and suitable individuals with a basic level of knowledge in varying building structures, heating systems and varying renewable technologies.

ILM Level 3 Certificate in Leadership & Management
By Challenge Consulting
ILM Level 3 Certificate in Leadership & Management – 9 day Accredited training course delivered in Nottingham A course for supervisors and junior managers which brings tangible benefits to the participants and to their organisations through applying the concepts taught at each stage directly to the work environment. The course is assessed in a practical manner through work based assignments for which tutorial guidance is supplied. The course is delivered in an interactive way to appeal to a variety of learning styles and to encourage participation.

FORS Safe Driving - Periodic 7 Hour CPC Course -Birmingham - Oct 2025
By Total Compliance
#SafeUrbanDriving #Birmingham #driver #driver_safety #driver_training #fors #nottingham #sud

An Understanding of fine-bore Nasogastric Tube Care and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for fine-bore nasogastric tube care and feeding with our healthcare professional course. Learn techniques, considerations, and patient care for enteral nutrition, medication administration, and gastric decompression.

Search By Location
- Practical Courses in London
- Practical Courses in Birmingham
- Practical Courses in Glasgow
- Practical Courses in Liverpool
- Practical Courses in Bristol
- Practical Courses in Manchester
- Practical Courses in Sheffield
- Practical Courses in Leeds
- Practical Courses in Edinburgh
- Practical Courses in Leicester
- Practical Courses in Coventry
- Practical Courses in Bradford
- Practical Courses in Cardiff
- Practical Courses in Belfast
- Practical Courses in Nottingham