- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Autism Diploma - Art & Play Therapy, Child Psychology and Mental Health - QLS Endorsed
4.8(9)By Skill Up
Level-4 QLS Endorsed | 30 in 1 Diploma Bundle | +Gifts: 30 Free CPD PDF Certificate | Lifetime Access

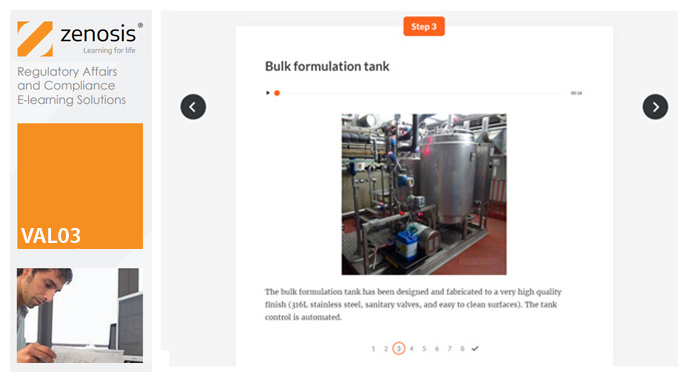
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

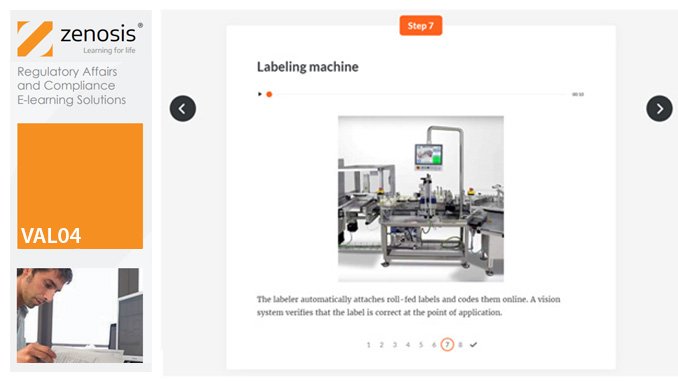
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Reiki Practitioner Level II (Okuden, Usui/Holy Fire III) ~ with Advanced Reiki Master, Sensei Alison
By British Reiki Circle
Open up a world of new possibilities with Reiki Practitioner Level II (Okuden). At this level you will be attunement to the 3 symbols using the Usui/Holy Fire ceremony tradition. Start your journey today with sensei Alison, Advanced Reiki Master and Crystal Reiki Master. Find out more about Alison, Reiki and the British Reiki Circle at: https://british-reiki-circle.cademy.co.uk/about

The online course is designed for people who like to learn Salsa or any Latin Dance rhythm at their own pace, time & space. For couples or individuals to achieve a good knowledge of the Cuban Salsa style. Taught by a native instructor. You can pay for the first lesson and have a taste of it then sign for the entire course which is made of another 5 more lesson Please make the payment and you will receive an email invitation to get access to the Course channel within the next 24 hours The Program includes: 1 or 6 Lesson Walking & Leading techniques Female & Male Teachers Footwork & Upper body techniques Steps & routines names (brain method) Body posture and language Tempo & beat music technique Fully equipped dance studio Ownership of your footage work progress & Docs Music library via Apple Music or Spotify Salsa or Latin Dance rhythm private class in London for couples or individuals to achieve a good knowledge of the salsa, samba, bachata, merengue or Latin dance steps One to One lesson, 4 minimum pack lesson book Teach by native instructor We have been training people in salsa in london for almost 20 years and still in business as salsa in west london is increasing massively.

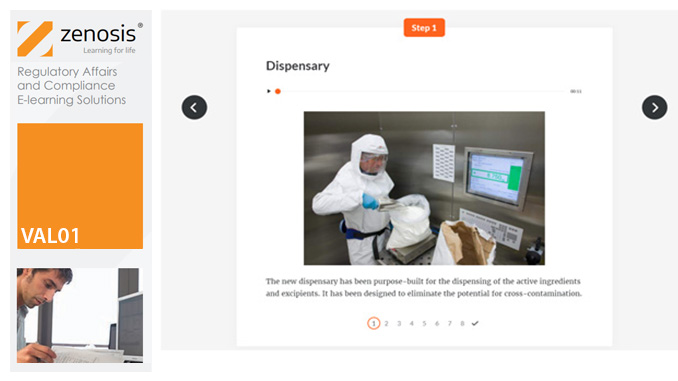
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
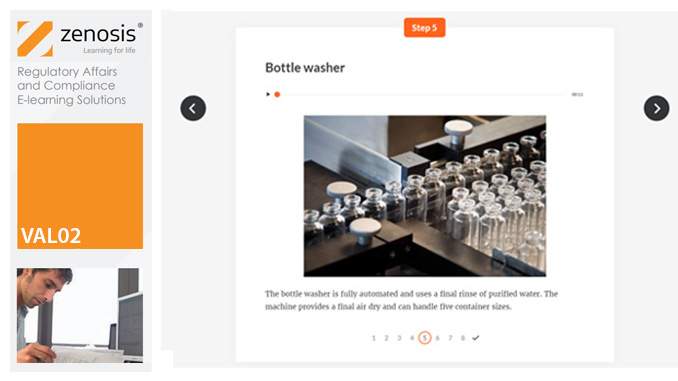
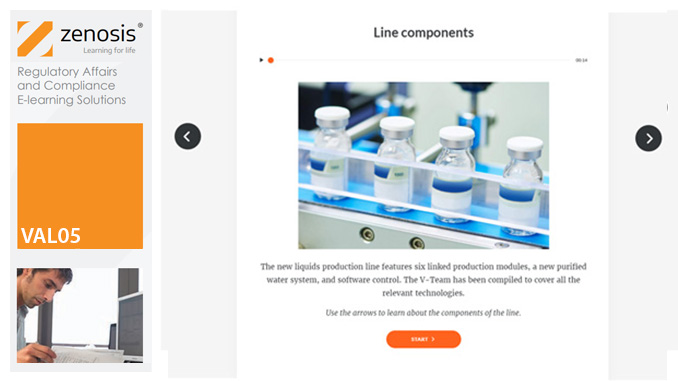
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.