- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7974 Systems courses
Day 1 of the Industrial Electrical Maintenance Part 1 consists of: Electricity at Work Regulations 1989, requirements and implementation Working safely, including the importance of isolation procedures and lock out systems Switches and Push Buttons, an explanation of the various types of switches and push buttons, the terminology and electrical symbols which are used in industry today, along with practical testing of functionality Relays and Contactors, principles of operation, purpose and uses Circuit design using BS electrical symbols and ladder diagrams, simple retaining circuits Construction of the above circuit, demonstrating the techniques of cable termination. Day 2 of the Industrial Electrical Maintenance Part 1 consists of: 3 phase induction motors, synchronous and asynchronous Description of the component parts of a 3 phase induction motor Demonstration of how a 3 phase rotating magnetic field is produced and how to reverse it Explanation of synchronous and asynchronous speed and slip Effect of the number of poles on motor speed Frame sizes Practical identification of various motors. Day 3 of the Industrial Electrical Maintenance Part 1 consists of: Principles of 3 phase induction motor control systems Overload protection principles of operation and use Design and operation of a DOL (direct on line) starter Construction of a DOL starter Inspection and testing procedure for the above starter Motor testing procedures. Day 4 of the Industrial Electrical Maintenance Part 1 consists of: DOL starter modifications Importance of updating documentation Design, construction and verification of reversing starter control and power circuits. Day 5 of the Industrial Electrical Maintenance Part 1 consists of: Star delta starters, principles of operation and uses Design, construction and verification of star delta starter control and power circuits.

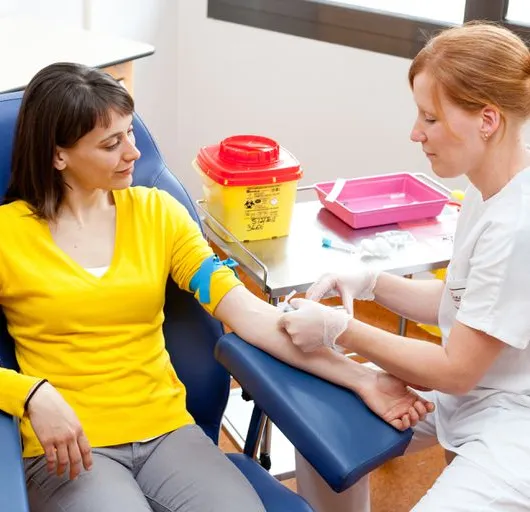
Learn how to take blood ... train as a Phlebotomist Nationally Recognised Qualification No previous experience or qualifications needed OCN Accredited - Level 3 (advanced) CPD Accredited (The CPD Certification Service) Covers all steps up to live blood draw Practise on artificial arm and fake blood! Basic understanding of English language required OPEN TO ALL APPLICANTS
This is an International Driver CPC - 7hours course - Vulnerable Road Users and it is suitable for LGV & PCV and will cover: Where Do We Encounter VRU’s? Who Are the VRU’s? Sharing Road Space, Route Planning, New Traffic Systems, Professional Drivers Role, Hazard Perception, Defensive Driving, Blindspots, Vehicle Safety Systems, Cyclists, Pedestrians, Motorcyclists, Horse Riders All courses start at 07:45 hrs and finish at 15:45 hrs All courses are 8 hours long with included 2 breaks of 15 minutes and a lunch break of 30 minutes. Approval: This course is registered with JAUPT as approved for Driver CPC qualification. Course Approval Number: ICRS5814/475. On completion of the course, all attendees will receive a certificate of attendance. Please note repeat courses are not accepted by DVSA and by joining this course you confirm that you are aware of the modules covered by you and certify that, if you have covered these before you are happy to repeat the modules due to needing further education on the subject.

Any person who requires the minimum legal requirement to work on air conditioning and refrigeration equipment including air conditioning and refrigeration engineers and technicians, all plant engineers, apprentices, personnel concerned with the recovery, charging or disposal of refrigerants and new comers into the industry. F Gas Regulations training courses are designed for both experienced air-conditioning and refrigeration engineers and also candidates who are looking to cross train and gain qualifications in the air conditioning / refrigeration sector. This F Gas regulations qualification is to meet the legal requirements of EC Regulation 842/2006 (commonly known as the F Gas Regulations). The Certification Schemes are designed for operatives who install, service and repair refrigeration, air-conditioning and heat pump systems, and require the following skills: Safe handling of refrigerant techniques Pipework and Jointing skills Recovery of Refrigerants (restricted to small systems only) Intrusive and non-intrusive leak and performance checking Category 1 covers all aspects of installation, commissioning, servicing, maintenance, recovery and leak checking on all refrigeration, air-conditioning and heat pump systems in accordance with the F-Gas Regulation EC842/2006, regardless of the weight of refrigerant in the system. F Gas regulations Course subjects are: Pressure test and discharge to BSEN378 Charge a blended refrigerant Evaluate system performance to ensure competence (using rule of thumb, temperature, refrigerant state and pressure) Leak test to EC1516/2007 Fabricate and fit brazed and mechanical joints Uphold F-Gas records and log books. Handle refrigerant safely and dispose of rightfully Pressure Regulation compliance All candidates will be able to ensure systems are tight, efficient in their use of energy and meet the F-Gas legal requirement.

A five day Refrigeration Electrics Maintenance course aimed at anyone involved with refrigeration, air conditioning and heat pump electrical control systems. The Refrigeration Electrics Maintenance course covers the three elements which are common to most refrigeration and air conditioning systems, namely, protection, control and motors. For example, a typical air conditioning split system will have protection provided by the fuse in the fused connection unit, and further protection provided to the cable feeding the fused connection unit, probably by a circuit breaker, control provided by thermostat and time clock etc… and motor driven fans and compressor. During the Refrigeration Electrics Maintenance course emphasis is also placed upon the applicable wiring regulations to ensure the constructed system is compliant with BS7671:2018 The aim of the Refrigeration Electrics Maintenance course is to provide enough knowledge to allow maintenance and fault finding to be safely and effectively carried out. The Refrigeration Electrics Maintenance course comprises of: Essential Electrics Module City and Guilds 2382-22 Level 3 – 18th Edition Wiring Regulations Refrigeration Electrics Module The Refrigeration Electrics Maintenance course costs include examination entry fees.

Search By Location
- Systems Courses in London
- Systems Courses in Birmingham
- Systems Courses in Glasgow
- Systems Courses in Liverpool
- Systems Courses in Bristol
- Systems Courses in Manchester
- Systems Courses in Sheffield
- Systems Courses in Leeds
- Systems Courses in Edinburgh
- Systems Courses in Leicester
- Systems Courses in Coventry
- Systems Courses in Bradford
- Systems Courses in Cardiff
- Systems Courses in Belfast
- Systems Courses in Nottingham