- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
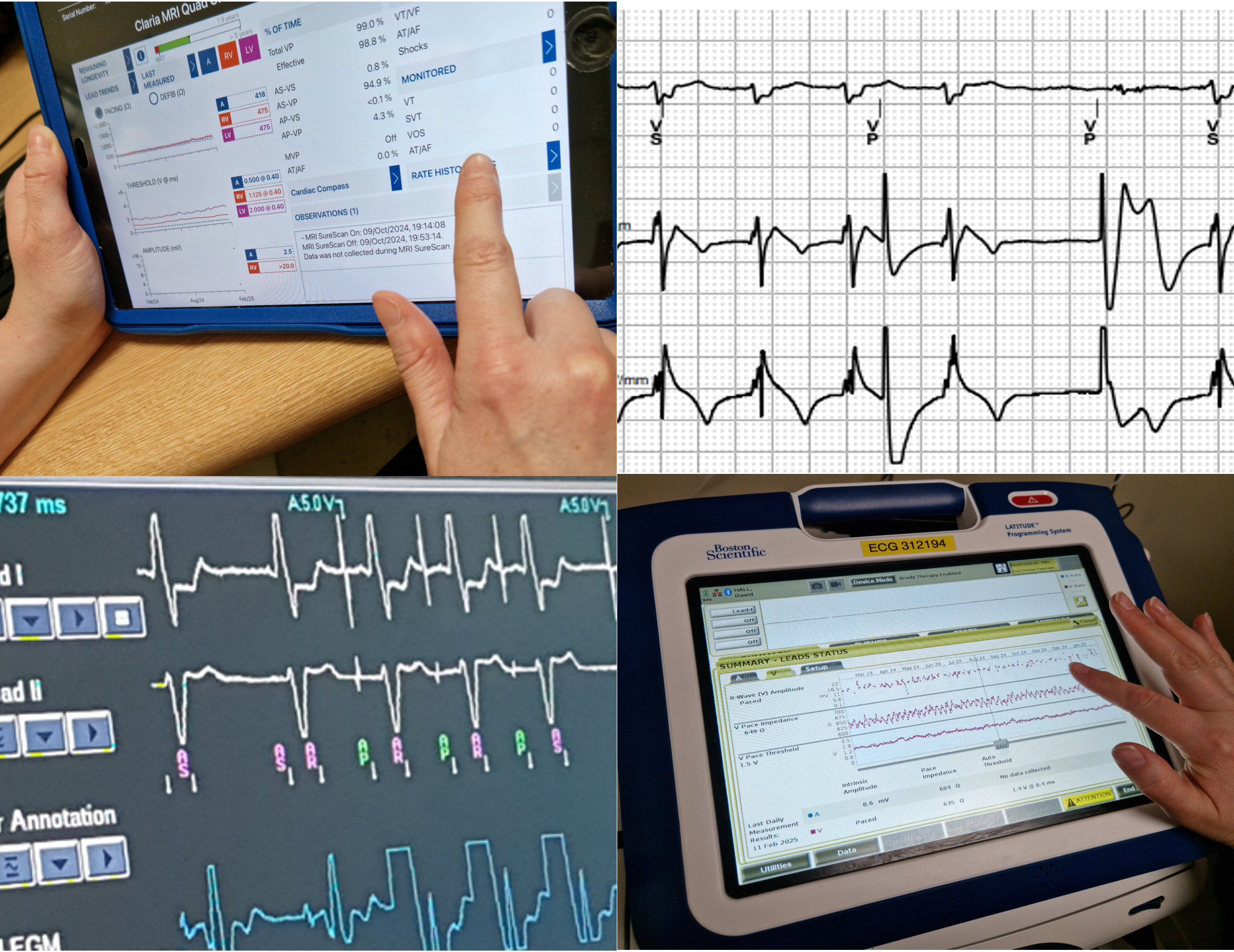
Demystifying cardiac devices — a practical introduction for healthcare professionals new to device therapy and monitoring A one day introductory course for all healthcare professionals with limited or no experience of cardiac devices. Ever wanted to know why there are so many different devices and what they do? What do device checks even involve? When are they needed? What should I be looking for on my telemetry? The course will be hosted by senior Cardiac Physiologists/ Scientists from the Cardiac Rhythm Management team at Manchester University NHS Foundation Trust. Full draft agenda below. **If submitting payment via CPD funding OR Purchase Order (i.e. not a card payment) - please DO complete your registration - you can detail payment method in the ‘Billing name' box. Many thanks. **
Advanced Load Forecasting & Methodology
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your forecasting skills with EnergyEdge's Advanced Load Forecasting Methodology course. Join our classroom training for expert insights.

NATIONALLY RECOGNISED AND ACCREDITED FORENSIC SCIENCE COURSE Level Three (advanced), awarding 3 credits. DUAL ACCREDITATION: Awarding Body: Open College Network (OCN Credit4Learning) Awarding Body: CPD (21 CPD Points) A modular "hybrid" forensic science course - eLearning (online) theory and one full day classroom based practical training (Crime Scene Investigations). The practical day covers a wide range of CSI techniques with "hands-on" practical training. On completion awards an OCN Nationally Recognised and Accredited Certificate in Forensic Science. This course is additionally CPD Accredited and also awards 21 CPD points on completion. PART 1 - THEORY Complete this part of your course online (eLearning Course) in the comfort of your own home or workplace. Please allow approx. 6-8 hours to complete the elearning modules. You do not need to complete Part 1 in one single "sitting” and can log on/off as many times as you wish and when convenient to you. You must complete both parts 1 and 2 to successfully pass this course. PART 2 - CLASSROOM Attend your forensic science practical day in the classroom, covering a number of key CSI investigative processes and procedures. You will develop your crime scene investigator skills with "hands-on" training in a classroom environment at the training location you have selected. Please note that we will provide protective clothing (disposable aprons), goggles and gloves. As you will be participating in a range of forensic activities we would suggest you wear easy clothing, short-sleeved top and closed-toe shoes. You can complete Part 1 before or after comleting Part 2. DOWNLOAD A COURSE ITINERARY HERE Course Itinerary M01 - Overview and Historical Background: A look at definitions, historical perspective highlighting major forensic advancements covering since early times, the beginning of modern forensics including the advent of fingerprinting, toxicology and DNA, and how DNA solved the first case (a double murder). Understanding the services of Forensic Labs and the major disciplines. M02 - Observational Skills Crime Scene Investigation & Recording Examination of the crime scene, photography, videography, sketch recording principles, using a CAD package. M03 - Forensic Pathology Understanding the job role, working within hospitals, mortuaries, the pathological processes and manner of death. M04 - Forensic Anthropology How does forensic anthropology help forensic scientists? Using physical markers present on a skeleton to determine age, sex, stature, and race. Bone anatomy and stages of development from foetal to elderly individual. Differentiating male and female: skull, pelvis, femur and humerus. M05 - Forensic Entomology How entomologists determine time of death as well as advanced investigations involving abuse and neglect. The life cycle of the blowfly and environmental influences. Using insect gut DNA to help solve crimes. CS01 - Case Study - Forensic Entomology - The Jigsaw Murders M06 - Forensic Serology Understanding presumptive tests and confirmatory tests. Tests in detail - processes and methods with options for: Blood, Saliva, Semen, Urine. M07 - Using the Microscope Correct procedures for using the light microscope. A look at the electron microscope and scanning probe microscope and their applications in forensic science. Detailed process guide including mounting slides.

Certified Artificial Intelligence Practitioner
By Mpi Learning - Professional Learning And Development Provider
This course shows you how to apply various approaches and algorithms to solve business problems through AI and ML, follow a methodical workflow to develop sound solutions, use open-source, off-the-shelf tools to develop, test, and deploy those solutions, and ensure that they protect the privacy of users. This course includes hands-on activities for each topic area.

Assessment Based Training – Python Programming & Analytics for the Oil & Gas Sector – Maximising Value from Data Assets
By EnergyEdge - Training for a Sustainable Energy Future
Maximize the value of data assets in the oil and gas sector with EnergyEdge's assessment-based training course on Python programming and analytics.

Hydraulic Fracturing: From Design to Execution and Post Frac Analysis
By EnergyEdge - Training for a Sustainable Energy Future
EnergyEdge course offers in-depth training on hydraulic fracturing. Learn about design, execution, and post-frac analysis. Enroll today!

Large Scale Hydrogen Production – Electrolyser Technologies & Electrolysis Interfaces
By EnergyEdge - Training for a Sustainable Energy Future
Unlock the future of energy with EnergyEdge's comprehensive classroom training on large-scale hydrogen production & electrolysis interfaces. Join us to revolutionize energy solutions!

Coal Power Plant Life Cycle Management and Flexible Operations in Energy Transition - Decommissioning, Preservation, Repurposing and Recommissioning
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge in coal power plant life cycle management and flexible operations with EnergyEdge. Learn about decommissioning, preservation, repurposing, and recommissioning.

Yield Assessment, Design and Monitoring of Solar Photovoltaic Power Plants – For Bankable System Simulations Using PVsyst Software
By EnergyEdge - Training for a Sustainable Energy Future
Learn to assess, design, and monitor solar PV power plants with EnergyEdge's comprehensive training. Master PVsyst software for bankable system simulations.

Search By Location
- Scientist Courses in London
- Scientist Courses in Birmingham
- Scientist Courses in Glasgow
- Scientist Courses in Liverpool
- Scientist Courses in Bristol
- Scientist Courses in Manchester
- Scientist Courses in Sheffield
- Scientist Courses in Leeds
- Scientist Courses in Edinburgh
- Scientist Courses in Leicester
- Scientist Courses in Coventry
- Scientist Courses in Bradford
- Scientist Courses in Cardiff
- Scientist Courses in Belfast
- Scientist Courses in Nottingham
