- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
13374 Professional Development courses
Overview Enrol in our Veterinary Nursing today and build the necessary skills, knowledge and experience to transform your career. The Veterinary Nursing could enhance your continuing professional development thus propelling you more towards your dream job. This Veterinary Nursing could be your key if you are interested in a long term career in the field of Veterinary Nursing. This Veterinary Nursing course consists of a number of easy to digest, in-depth modules which are designed to provide you with detailed knowledge on Veterinary Nursing. This Veterinary Nursing aims to accompany you through your journey to help you become a master of Veterinary Nursing. Learn through a mixture of interactive lessons and online study materials. How will I get my certificate? At the end of the course there will be a written assignment test which you can take either during or after the course. After successfully completing the test you will be able to order your certificate, these are included in the price. Who is this course for? There is no experience or previous qualifications required for enrolment in this Veterinary Nursing. It is available to all students, of all academic backgrounds. Requirements Our Veterinary Nursing is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G.There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business &Management , Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 3 sections • 19 lectures • 04:05:00 total length •Getting to Know the Kittens and Cats: 00:28:00 •Collapse & Unconsciousness: 00:12:00 •Cardiopulmonary Resuscitation: 00:21:00 •Shock: 00:11:00 •Bleeding: 00:15:00 •Bandaging: 00:27:00 •Fractures: 00:09:00 •Seizures: 00:05:00 •Choking & Drowning: 00:07:00 •Heat Stroke & Hypothermia: 00:05:00 •Burns & Scalds: 00:06:00 •Poisoning: 00:10:00 •Bites & Stings: 00:05:00 •Common Emergencies: 00:17:00 •Managing a First Aid Scenario: 00:05:00 •Pet First Aid Kit: 00:10:00 •Pet CPR: 00:12:00 •Mock Exam - Veterinary Nursing: 00:20:00 •Final Exam - Veterinary Nursing: 00:20:00

ICT01: Compliance with Regulation 21 CFR Part 11 on Electronic Records and Electronic Signatures
By Zenosis
21CFR11 applies to records that are required to be submitted to the FDA, or that are subject to FDA inspection, and that are in electronic form – that is, as computer files. It applies to all computer systems used to create, modify, maintain, archive, retrieve, or transmit such records – from a humble spreadsheet program to a complex information management system.

PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

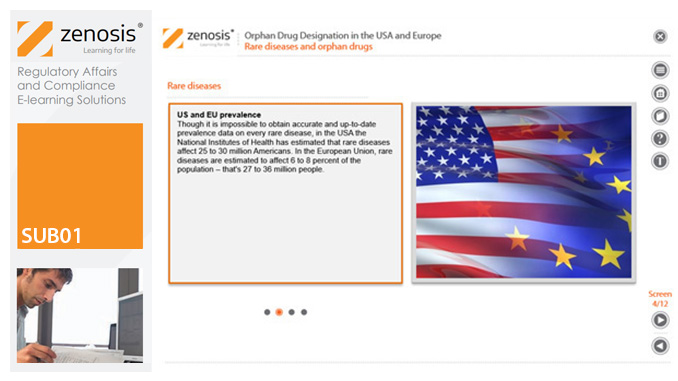
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
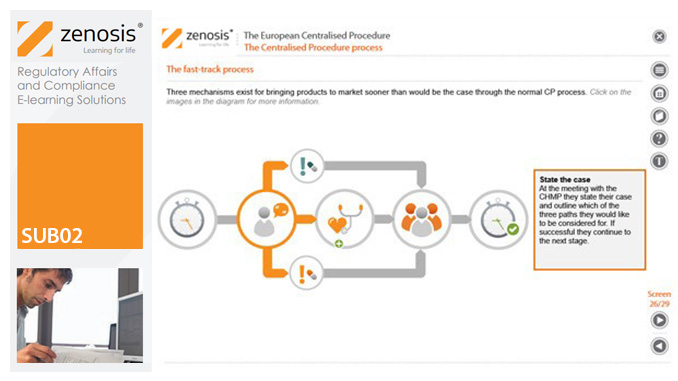
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
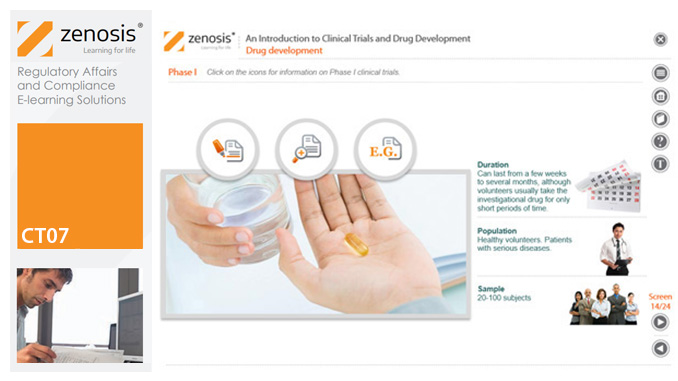
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
Search By Location
- Professional Development Courses in London
- Professional Development Courses in Birmingham
- Professional Development Courses in Glasgow
- Professional Development Courses in Liverpool
- Professional Development Courses in Bristol
- Professional Development Courses in Manchester
- Professional Development Courses in Sheffield
- Professional Development Courses in Leeds
- Professional Development Courses in Edinburgh
- Professional Development Courses in Leicester
- Professional Development Courses in Coventry
- Professional Development Courses in Bradford
- Professional Development Courses in Cardiff
- Professional Development Courses in Belfast
- Professional Development Courses in Nottingham