- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11580 Professional Development courses in Reading delivered Online
Mastering Ohm's Law for Series-Parallel Circuits
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. Course Curriculum Introduction Three Types of Circuits 00:06:00 Calculating Total Resistance in Series Parallel Circuits Calculating Total Resistance in Series Parallel Circuits - Example 1 00:04:00 Redrawing the Picture for Easier Understanding 00:02:00 Six Resistor Example Explained 00:04:00 Total Resistance Calculation - Example 2 00:03:00 Total Resistance Calculation - Example 3 00:02:00 Total Resistance Calculation - Example 4 00:03:00 Total Resistance Calculation - Example 5 00:03:00 Electrically Equivalent Pictures and Total Resistance Review 00:05:00 Series Circuits. Parallel Circuits and Ohm's Law Review Series Practice Example Revisited 00:08:00 Parallel Practice Example Revisited 00:05:00 Ohm's Law Review 00:01:00 Ohms Law Formulas 00:03:00 Power Formula Review 00:01:00 Series Parallel Math Example Series Parallel - Ohm's Law Example 1 00:10:00 Series Parallel - Ohm's Law Example 2 00:06:00 Series Parallel - Ohm's Law Example 3 00:12:00 Series Parallel - Ohm's Law Example 4 00:10:00 Series Parallel Demo Section Series Parallel Demo 1 00:04:00 Series Parallel Demo 2 00:04:00 Series Parallel Demo 3 00:07:00 Thank you and Keep in Touch 00:01:00 Supplementary Resources Supplementary Resources - Ohm's Law Ohm's Law Made Easy for Series Parallel Circuits 00:00:00 Obtain Your Certificate Order Your Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00
Powershell Training for Microsoft Office 365
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. Course Curriculum Introduction Introduction 00:02:00 PowerShell Basic Introduction PowerShell Introduction 00:05:00 PowerShell Console (PowerShell ISE, VS Code) 00:05:00 PowerShell Useful Commands (Get-Help) 00:04:00 PowerShell Useful Commands (Get-Command) 00:01:00 PowerShell Useful Commands (Get-Module) 00:03:00 Visual Studio Code Editor Initial Setup Visual Studio Code Setup 00:02:00 VS Code Interface & Install PowerShell Ext 00:03:00 Write & Test PowerShell Script 00:02:00 Connect To Azure Active Directory through Powershell Azure Active Directory Introduction 00:04:00 Prerequisite & Install AZURE AD PS Module 00:03:00 Connect to Azure AD PowerShell with Microsoft Office 365 Subscription 00:04:00 Install All Office 365 Service's Module (To Prepare All-in-one Scripts) 00:03:00 All-In-One PowerShell Script To Connect All M365 Services 00:09:00 Administration Of Microsoft Office 365 User Accounts View Microsoft Office 365 User Accounts 00:05:00 Create Single Microsoft Office 365 User Account 00:09:00 Create Multiple Microsoft Office 365 User Accounts 00:04:00 Modify Microsoft Office 365 User Account Properties 00:08:00 Delete Microsoft Office 365 User Accounts 00:03:00 Block Microsoft Office 365 User Accounts 00:02:00 Administration Of Microsoft Office 365 Licenses And Service View M365 Licenses Details 00:04:00 View M365 License's Services Details 00:03:00 Assign Licenses to Single User Account (Older AzureAD Module) 00:02:00 Assign Licenses to Multiple User Account 00:02:00
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

VAL05: Equipment Cleaning Validation
By Zenosis
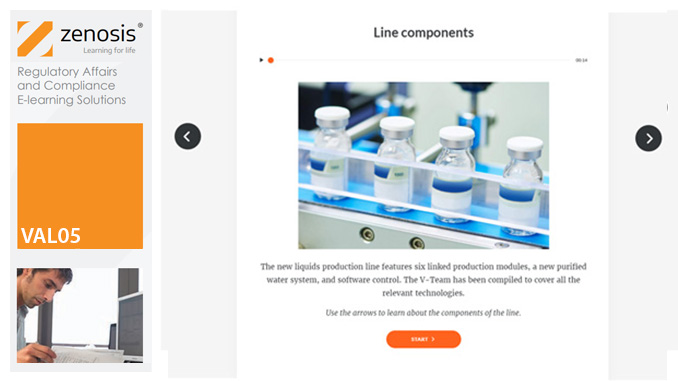
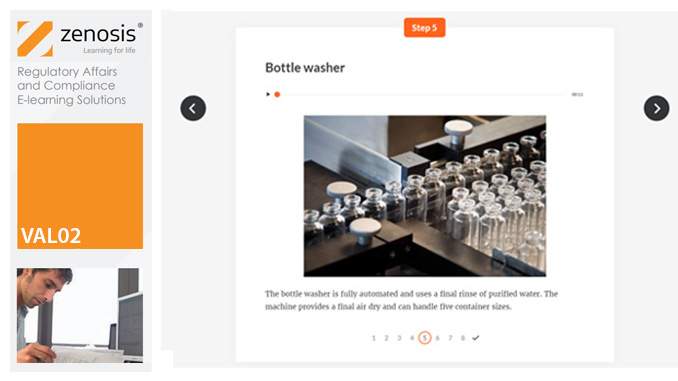
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL02: Validation Plans and Documentation
By Zenosis
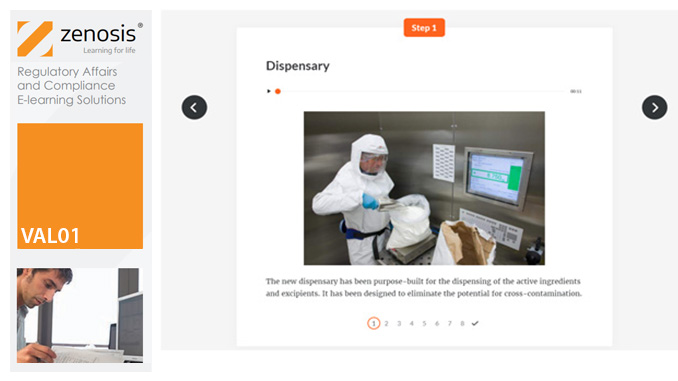
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
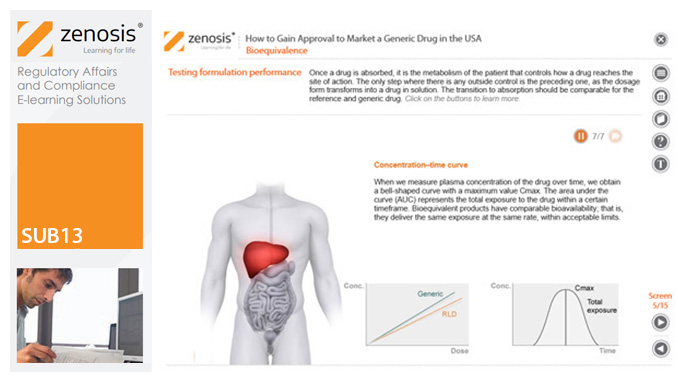
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.