- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11828 Professional Development courses in Perth delivered Online
Personal Trainer Courses Online Mega Bundle
By Study Plex
Highlights of the Course Course Type: Online Learning Duration: 360 Hours Tutor Support: Tutor support is included Customer Support: 24/7 customer support is available Quality Training: The course is designed by an industry expert Recognised Credential: Recognised and Valuable Certification Completion Certificate: Free Course Completion Certificate Included Instalment: 3 Installment Plan on checkout What you will learn from this course? Gain comprehensive knowledge about fitness trainer Understand the core competencies and principles of fitness trainer Explore the various areas of fitness trainer Know how to apply the skills you acquired from this course in a real-life context Become a confident and expert fitness trainer Personal Trainer Courses Online Mega Bundle Are you interested to begin your personal trainer career or want to gain more advanced skills in personal trainer? Then this personal trainer courses online course will help you get one step closer to your professional aspirations and develop your skills and knowledge in personal trainer for a rewarding career. This Bundle Package Includes: The skills and knowledge that you will gain through studying the following courses will teach you the theory of effective personal trainer practice and equip you with the essential skills, confidence and competence to assist you in the personal trainer industry. You'll gain a solid understanding of the core competencies required to drive a successful career in personal trainer. Fitness Trainer Course Six Pack Abs & Muscle Building Muscle Building - Bodyweight Workout Bodyweight Workout Fitness and Weight Loss Nutrition - Healthy Eating & Diet Planning Fitness and Health Weight Loss Mastery Nutrition - Diet For Faster Weight Loss Fitness Trainer Course Diet and Nutrition Masterclass Health and Wellness Coaching Gain personal trainer skills and credentials that employers value. This bundle course is accredited by the CPD UK. CPD is globally recognised by employers, professional organisations and academic intuitions, thus a certificate from CPD Certification Service creates value towards to your professional goal and achievement. CPD certified certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Master the skills you need to propel your career forward in personal trainer. Equip yourself with the essential knowledge and skillset that make you a confident personal trainer and take your career to the next level. This comprehensive bundle course is designed to help you reach your professional goals. Learn from expert tutors with industry experience, teaching you the latest expertise and best practice. This extensive course is designed for personal trainer professionals who are aspiring to specialise in personal trainer. Earn industry-recognised credentials to demonstrate your new skills and add extra value to your CV. Enrol today and take the next step towards your personal and professional goals. Who is this Course for? This comprehensive personal trainer course is ideal for anyone wishing to boost their career profile or advance their career in this field by gaining a thorough understanding of the subject. Anyone willing to gain extensive knowledge on this fitness trainer can also take this course. Whether you are a complete beginner or an aspiring professional, this course will provide you with the necessary skills and professional competence, and open your doors to a wide number of professions within your chosen sector. Entry Requirements This personal trainer course has no academic prerequisites and is open to students from all academic disciplines. You will, however, need a laptop, desktop, tablet, or smartphone, as well as a reliable internet connection. Assessment This personal trainer course assesses learners through multiple-choice questions (MCQs). Upon successful completion of the modules, learners must answer MCQs to complete the assessment procedure. Through the MCQs, it is measured how much a learner could grasp from each section. In the assessment pass mark is 60%. Advance Your Career This personal trainer course will provide you with a fresh opportunity to enter the relevant job market and choose your desired career path. Additionally, you will be able to advance your career, increase your level of competition in your chosen field, and highlight these skills on your resume. Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. What is CPD? Employers, professional organisations, and academic institutions all recognise CPD, therefore a credential from CPD Certification Service adds value to your professional goals and achievements. Benefits of CPD Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market Demonstrate your dedication Showcases your professional capabilities What is IPHM? The IPHM is an Accreditation Board that provides Training Providers with international and global accreditation. The Practitioners of Holistic Medicine (IPHM) accreditation is a guarantee of quality and skill. Benefits of IPHM It will help you establish a positive reputation in your chosen field You can join a network and community of successful therapists that are dedicated to providing excellent care to their client You can flaunt this accreditation in your CV It is a worldwide recognised accreditation What is Quality Licence Scheme? This course is endorsed by the Quality Licence Scheme for its high-quality, non-regulated provision and training programmes. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. Benefits of Quality License Scheme Certificate is valuable Provides a competitive edge in your career It will make your CV stand out Course Curriculum Fitness Trainer Course Six Pack Abs & Muscle Building Muscle Building - Bodyweight Workout Bodyweight Workout Fitness and Weight Loss Nutrition - Healthy Eating & Diet Planning Fitness and Health Weight Loss Mastery Nutrition - Diet For Faster Weight Loss Fitness Trainer Course Diet and Nutrition Masterclass Health and Wellness Coaching Obtain Your Certificate Order Your Certificate of Achievement 00:00:00

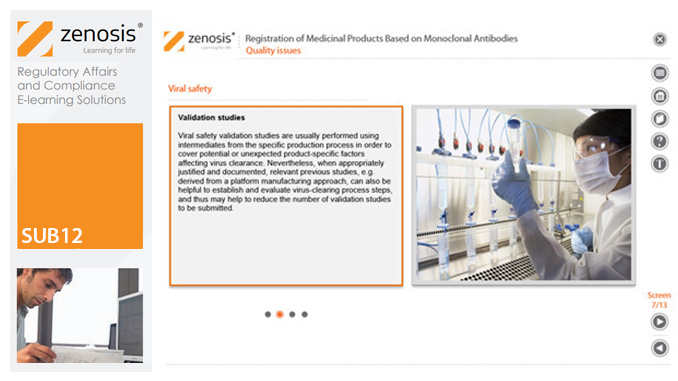
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

PKPD02: Conducting Pharmacokinetic and Pharmacodynamic Studies
By Zenosis
This module extends the learner’s understanding of pharmacokinetic and pharmacodynamic studies from the basics described in our companion module PKPD01, An Introduction to Pharmacokinetics and Pharmacodynamics in Drug Development and Registration. It provides detail on a variety of aspects of such studies: design, sampling, data analysis, research in special populations, and bioequivalence testing.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
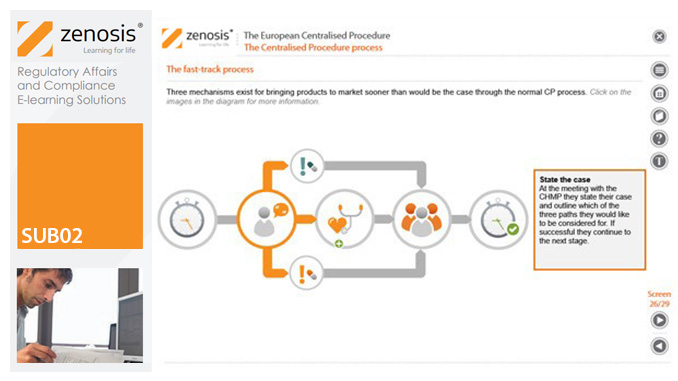
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.
CT06: Clinical Trial Monitoring: Site Evaluation and Setup
By Zenosis
The sponsor of a clinical trial needs to reach agreement with clinical investigators to conduct the trial. The suitability of investigators and their institutional sites, typically hospitals, has to be evaluated, and the trial has to be set up at each site. This module describes the processes involved, focusing particularly on the role of a Clinical Research Associate (CRA) employed or contracted by the sponsor to monitor the trial.
CT07: An Introduction to Clinical Trials and Drug Development
By Zenosis
This module provides an understanding of how clinical trials fit into the drug development process. It outlines the key historical events leading to the development of controlled clinical trials. It specifies the purpose of trials, outlines their features, and identifies codes and regulations that apply to them. Finally, it describes the environment of cost control in which the modern pharmaceutical industry operates.
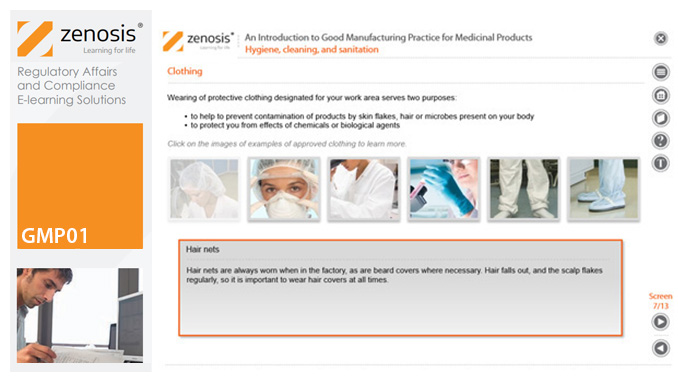
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.