- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11746 Professional Development courses in London delivered Online
Child Psychology, Child Care, Child Protection, Mental Health & Behaviour- 20 Courses Bundle
By NextGen Learning
Get ready for an exceptional online learning experience with the Child Psychology, Child Care, Child Protection, Mental Health & Behaviour bundle! This carefully curated collection of 20 premium courses is designed to cater to a variety of interests and disciplines. Dive into a sea of knowledge and skills, tailoring your learning journey to suit your unique aspirations. Child Psychology, Child Care, Child Protection, Mental Health & Behaviour is a dynamic package that blends the expertise of industry professionals with the flexibility of digital learning. It offers the perfect balance of foundational understanding and advanced insights. Whether you're looking to break into a new field or deepen your existing knowledge, the Child Psychology, Child Care & Child Protection package has something for everyone. As part of the Child Psychology, Child Care, Child Protection, Mental Health & Behaviour package, you will receive complimentary PDF certificates for all courses in this bundle at no extra cost. Equip yourself with the Child Psychology, Child Care & Child Protection bundle to confidently navigate your career path or personal development journey. Enrol today and start your career growth! This Bundle Comprises the Following CPD Accredited Courses: Child Psychology Diploma Child Development and Care Montessori and Waldorf Approaches for Early Childhood Child Behaviour Children's Mental Health Child Neglect Awareness Certificate Course Cognitive and Social Development at Early Childhood Using Reggilio Emilia Approach in Early Childhood Socialisation and Adaptation in Early Childhood Safeguarding children level 3 Child Attachment Diploma Child Protection & Safeguarding Child Playwork Creating Children's Room How to Handle Tantrums in Children Career Development Plan Fundamentals CV Writing and Job Searching Learn to Level Up Your Leadership Networking Skills for Personal Success Ace Your Presentations: Public Speaking Masterclass Learning Outcome: Gain comprehensive insights into multiple fields. Foster critical thinking and problem-solving skills across various disciplines. Understand industry trends and best practices through the Child Psychology, Child Care & Child Protection Bundle. Develop practical skills applicable to real-world situations. Enhance personal and professional growth with the Child Psychology, Child Care & Child Protection Bundle. Build a strong knowledge base in your chosen course via the Child Psychology, Child Care & Child Protection Bundle. Benefit from the flexibility and convenience of online learning. With the Child Psychology, Child Care & Child Protection package, validate your learning with a CPD certificate. Each course in this Child Psychology, Child Care & Child Protection bundle holds a prestigious CPD accreditation, symbolising exceptional quality. The materials, brimming with knowledge, are regularly updated, ensuring their relevance. This bundle promises not just education but an evolving learning experience. Engage with this extraordinary collection, and prepare to enrich your personal and professional development. Embrace the future of learning with Child Psychology, Child Care, Child Protection, Mental Health & Behaviour, a rich anthology of 15 diverse courses. Each course in the Child Psychology, Child Care & Child Protection bundle is handpicked by our experts to ensure a wide spectrum of learning opportunities. This Child Psychology, Child Care, Child Protection, Mental Health & Behaviour bundle will take you on a unique and enriching educational journey. The bundle encapsulates our mission to provide quality, accessible education for all. Whether you are just starting your career, looking to switch industries, or hoping to enhance your professional skill set, the Child Psychology, Child Care, Child Protection, Mental Health & Behaviour bundle offers you the flexibility and convenience to learn at your own pace. Make the Child Psychology, Child Care & Child Protection package your trusted companion in your lifelong learning journey. CPD 240 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Child Psychology, Child Care, Child Protection, Mental Health & Behaviour bundle is perfect for: Lifelong learners are looking to expand their knowledge and skills. Professionals are seeking to enhance their careers with CPD certification. Individuals wanting to explore new fields and disciplines. Anyone who values flexible, self-paced learning from the comfort of home. Requirements Without any formal requirements, anyone can enrol in this Child Psychology, Child Care & Child Protection course easily. Career path Unleash your potential with the Child Psychology, Child Care, Child Protection, Mental Health & Behaviour bundle. Acquire versatile skills across multiple fields, foster problem-solving abilities, and stay ahead of industry trends. Ideal for those seeking career advancement, a new professional path, or personal growth. Embrace the journey with this bundle package. Certificates Certificate Of Completion Digital certificate - Included Certificate Of Completion Hard copy certificate - Included You will get a complimentary Hard Copy Certificate.

Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR - 20 Courses Bundle
By NextGen Learning
Get ready for an exceptional online learning experience with the Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR bundle! This carefully curated collection of 20 premium courses is designed to cater to a variety of interests and disciplines. Dive into a sea of knowledge and skills, tailoring your learning journey to suit your unique aspirations. The Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR is a dynamic package, blending the expertise of industry professionals with the flexibility of digital learning. It offers the perfect balance of foundational understanding and advanced insights. Whether you're looking to break into a new field or deepen your existing knowledge, the Human Rights package has something for everyone. As part of the Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR package, you will receive complimentary PDF certificates for all courses in this bundle at no extra cost. Equip yourself with the Human Rights bundle to confidently navigate your career path or personal development journey. Enrol today and start your career growth! This Bundle Comprises the Following CPD Accredited Courses: Human Rights Equality, Diversity and Discrimination Cross-Cultural Awareness Training Social Inclusion Officer Training Course Unconscious Bias Training Course Domestic Violence and Abuse Awareness Diploma Workplace Harassment Workplace Confidentiality Youth Work & Leadership Preventing Radicalisation and Extremism Inspirational Leadership Skills: Practical Motivational Leadership Organisation & People Management Leadership and Management Course PR : Public Relation Communication Masterclass Building Strong Relationships Career Development Plan Fundamentals CV Writing and Job Searching Learn to Level Up Your Leadership Networking Skills for Personal Success Ace Your Presentations: Public Speaking Masterclass Learning Outcome: Gain comprehensive insights into multiple fields. Foster critical thinking and problem-solving skills across various disciplines. Understand industry trends and best practices through the Human Rights Bundle. Develop practical skills applicable to real-world situations. Enhance personal and professional growth with the Human Rights. Build a strong knowledge base in your chosen course via the Human Rights. Benefit from the flexibility and convenience of online learning. With the Human Rights package, validate your learning with a CPD certificate. Each course in this bundle holds a prestigious CPD accreditation, symbolising exceptional quality. The materials, brimming with knowledge, are regularly updated, ensuring their relevance. This bundle promises not just education but an evolving learning experience. Engage with this extraordinary collection, and prepare to enrich your personal and professional development. Embrace the future of learning with the Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR, a rich anthology of 15 diverse courses. Each course in the Human Rights bundle is handpicked by our experts to ensure a wide spectrum of learning opportunities. ThisHuman Rights, Equality & Diversity, Abuse Awareness, Leadership & PR bundle will take you on a unique and enriching educational journey. The bundle encapsulates our mission to provide quality, accessible education for all. Whether you are just starting your career, looking to switch industries, or hoping to enhance your professional skill set, the Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR bundle offers you the flexibility and convenience to learn at your own pace. Make the Human Rights package your trusted companion in your lifelong learning journey. CPD 200 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR bundle is perfect for: Lifelong learners looking to expand their knowledge and skills. Professionals seeking to enhance their career with CPD certification. Individuals wanting to explore new fields and disciplines. Anyone who values flexible, self-paced learning from the comfort of home. Career path Unleash your potential with the Human Rights, Equality & Diversity, Abuse Awareness, Leadership & PR bundle. Acquire versatile skills across multiple fields, foster problem-solving abilities, and stay ahead of industry trends. Ideal for those seeking career advancement, a new professional path, or personal growth. Embrace the journey with the Human Rightsbundle package. Certificates Certificate Of Completion Digital certificate - Included Certificate Of Completion Hard copy certificate - Included You will get a complimentary Hard Copy Certificate.

Painter and Decorator Colour Mixing Course for Acrylice and Oil Painters Online
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. What is CPD? Employers, professional organisations, and academic institutions all recognise CPD, therefore a credential from CPD Certification Service adds value to your professional goals and achievements. Benefits of CPD Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market Demonstrate your dedication Showcases your professional capabilities What is IPHM? The IPHM is an Accreditation Board that provides Training Providers with international and global accreditation. The Practitioners of Holistic Medicine (IPHM) accreditation is a guarantee of quality and skill. Benefits of IPHM It will help you establish a positive reputation in your chosen field You can join a network and community of successful therapists that are dedicated to providing excellent care to their client You can flaunt this accreditation in your CV It is a worldwide recognised accreditation What is Quality Licence Scheme? This course is endorsed by the Quality Licence Scheme for its high-quality, non-regulated provision and training programmes. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. Benefits of Quality License Scheme Certificate is valuable Provides a competitive edge in your career It will make your CV stand out Course Curriculum New Color Mixing for Painters - in Acrylic or Oil Introduction 00:09:00 Materials and Preparing Your Color Palette 00:21:00 Mixing Purples - And All Secondary Colors 00:19:00 Mixing Reds - And All Primary Colors 00:10:00 Mixing Neutral Colors 00:11:00 More Tips on Color 00:27:00 Certificate of Achievement Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00

Renewable Energy Basics
By Study Plex
Recognised Accreditation This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. What is CPD? Employers, professional organisations, and academic institutions all recognise CPD, therefore a credential from CPD Certification Service adds value to your professional goals and achievements. Benefits of CPD Improve your employment prospects Boost your job satisfaction Promotes career advancement Enhances your CV Provides you with a competitive edge in the job market Demonstrate your dedication Showcases your professional capabilities What is IPHM? The IPHM is an Accreditation Board that provides Training Providers with international and global accreditation. The Practitioners of Holistic Medicine (IPHM) accreditation is a guarantee of quality and skill. Benefits of IPHM It will help you establish a positive reputation in your chosen field You can join a network and community of successful therapists that are dedicated to providing excellent care to their client You can flaunt this accreditation in your CV It is a worldwide recognised accreditation What is Quality Licence Scheme? This course is endorsed by the Quality Licence Scheme for its high-quality, non-regulated provision and training programmes. The Quality Licence Scheme is a brand of the Skills and Education Group, a leading national awarding organisation for providing high-quality vocational qualifications across a wide range of industries. Benefits of Quality License Scheme Certificate is valuable Provides a competitive edge in your career It will make your CV stand out Course Curriculum Renewable Energy Introduction 00:03:00 Current status and trends 00:13:00 solar energy 00:12:00 Wind energy 00:11:00 Bioenergy 00:10:00 Geothermal energy 00:08:00 Hydro Energy 00:09:00 Assessment Assessment - Renewable Energy Basics 00:10:00 Certificate of Achievement Certificate of Achievement 00:00:00 Get Your Insurance Now Get Your Insurance Now 00:00:00 Feedback Feedback 00:00:00

Teaching English: 120 hours TEFL / TESOL - CPD Certified
By Compliance Central
***Teaching English: TEFL / TESOL - Become a Qualified English Language Teacher!*** The demand for qualified English language teachers (TEFL/TESOL) is on the rise! A recent industry report states that the global English language learning market is expected to reach $20.1 billion by 2027. This TEFL/TESOL course equips you with the necessary knowledge and theoretical foundation to embark on a rewarding career teaching English to speakers of other languages (ESOL) around the world. Our Teaching English course starts with the basics of Teaching English and gradually progresses towards advanced topics. Therefore, each lesson of this Teaching English course is intuitive and easy to understand. Learning Outcomes By the end of this Teaching English: TEFL / TESOL course, you will be able to: Demonstrate a strong understanding of the principles of Teaching English: TEFL / TESOL. Apply a variety of effective teaching methodologies to different learning styles. Plan, develop, and deliver engaging and interactive English language lessons. Create effective teaching materials to support student learning. Manage a classroom environment that fosters motivation and participation. Teaching English: TEFL / TESOL professionally and ethically. Why would you choose the Teaching English: TEFL / TESOL course: Lifetime access to Teaching English: TEFL / TESOL course materials Full tutor support is available from Monday to Friday with the Teaching English: TEFL / TESOL course Learn Teaching English: TEFL / TESOL skills at your own pace from the comfort of your home Gain a complete understanding of Teaching English: TEFL / TESOL course Accessible, informative Teaching English: TEFL / TESOL learning modules designed by experts Get 24/7 help or advice from our email and live chat teams with the Study Teaching English: TEFL / TESOL in your own time through your computer, tablet or mobile device A 100% learning satisfaction guarantee with your Teaching English: TEFL / TESOL course Free Courses: Course 01: Teaching Strategies for Remote Classroom Course 02: Lesson Planning for Teaching This Teaching English: TEFL / TESOL course is designed to provide a thorough exploration of the theoretical underpinnings of effective English language instruction. Modules 1-3: Establish a solid foundation in Teaching English: TEFL / TESOL by exploring core concepts like language learning theories, linguistics principles, and English pronunciation. Modules 4-5: Delve into the art of teaching grammar and vocabulary effectively in Teaching English: TEFL / TESOL contexts. Gain insights into various language teaching methodologies that can be applied in the classroom. Modules 6-9: Focus on the development of all four language skills: reading, writing, listening, and speaking. Learn strategies for effective teaching of each skill specific to Teaching English: TEFL / TESOL learners. Modules 10-12: Equip yourself with the tools to create engaging and well-structured lesson plans and explore the applications of technology in Teaching English: TEFL / TESOL classrooms. Modules 13-14: Discover effective classroom management techniques and explore the situational approach in Teaching English: TEFL / TESOL. Module 15: Conclude your journey by exploring avenues for continuous professional development and opportunities within the Teaching English: TEFL / TESOL field. Curriculum Breakdown of Teaching English: 120 hours TEFL / TESOL - CPD Certified Course Module 01: Introduction to English Language Teaching & Learning Module 02: Linguistics Module 03: English Pronunciation Module 04: Teaching Grammar and Vocabulary Module 05: Language Teaching Methodologies Module 06: Teaching Receptive Skills: Reading Module 07: Teaching Productive Skills: Writing Module 08: Teaching Receptive Skills: Listening Module 09: Teaching Productive Skills: Speaking Module 10: Lesson Planning and Creating Materials Module 11: Use of Games and Activities Module 12: Technology in Second Language Teaching & Learning Module 13: Classroom Management and Student Motivation Module 14: Teaching English: Situational Approach Module 15: Professional Development and Employment CPD 10 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Teaching English: TEFL / TESOL course is ideal for: Individuals with no prior teaching experience who are interested in a career in Teaching English: TEFL / TESOL. Educators who want to gain a strong theoretical foundation in Teaching English: TEFL / TESOL principles. Professionals seeking to transition into the field of Teaching English: TEFL / TESOL. Anyone passionate about languages and interested in Teaching English: TEFL / TESOL to speakers of other languages. Travellers who want to teach English abroad and gain valuable cultural experiences. Individuals looking to enhance their employability and qualifications. Requirements There are no prior teaching qualifications required to enroll in this Teaching English: TEFL / TESOL course. A passion for languages and a desire to teach English to speakers of other languages are essential. pen_spark Career path The Teaching English course will enhance your knowledge and improve your confidence in exploring opportunities in various sectors. ESL Teacher: £22,000 to £51,000 per year English Language Instructor: £29,000 to £58,000 per year Teacher Trainer: £36,000 to £72,000 per year Academic Director: £43,000 to £86,000 per year Language Center Manager: £36,000 to £72,000 per year Certificates 3 CPD Accredited PDF Certificate Digital certificate - Included CPD Accredited PDF Certificate Each CPD Accredited Hard Copy Certificate Hard copy certificate - £10.79 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

Unlock the power of transformative mentorship with our 'Intermediate Guide on Motivational Mentorship' course. Elevate your mentorship skills, foster strong mentor-mentee relationships, and navigate challenges in a diverse and digital landscape. Learn ethical considerations, set SMART goals, and measure success. Ideal for professionals and educators seeking to inspire personal and professional growth. Join us on a journey of empowerment and discover the future of mentorship!

Revit Architecture and Construction Management Fundamentals - 20 Courses Bundle
By NextGen Learning
Get ready for an exceptional online learning experience with the Revit Architecture and Construction Management Fundamentals bundle! This carefully curated collection of 20 premium courses is designed to cater to a variety of interests and disciplines. Dive into a sea of knowledge and skills, tailoring your learning journey to suit your unique aspirations. The Revit Architecture and Construction Management Fundamentals is a dynamic package, that blends the expertise of industry professionals with the flexibility of digital learning. It offers the perfect balance of foundational understanding and advanced insights. Whether you're looking to break into a new field or deepen your existing knowledge, the Construction Management package has something for everyone. As part of the Revit Architecture and Construction Management Fundamentals package, you will receive complimentary PDF certificates for all courses in this bundle at no extra cost. Equip yourself with the Construction Management bundle to confidently navigate your career path or personal development journey. Enrol today and start your career growth! This Bundle Comprises the Following CPD Accredited Courses: Revit Architecture Ultimate Interior Design Course Revit Architecture Ultimate Exterior Design for Beginners Revit Architecture Advanced Interior Design Training Construction Management Construction Cost Estimation Construction Safety Intermediate Solidworks Course Finite Element Analysis Using Solidworks Solidworks: Beginner to Advanced Solidworks: Complete CSWA Preparation Solidworks Foundation Training Solidworks Drawing Tools Training: Test Preparation Solidworks: CSWA Exam Preparation Solidworks: CSWP Exam Preparation Architectural Studies Career Development Plan Fundamentals CV Writing and Job Searching Learn to Level Up Your Leadership Networking Skills for Personal Success Ace Your Presentations: Public Speaking Masterclass Learning Outcome: Gain comprehensive insights into multiple fields. Foster critical thinking and problem-solving skills across various disciplines. Understand industry trends and best practices through the Construction Management Bundle. Develop practical skills applicable to real-world situations. Enhance personal and professional growth with the Construction Management Bundle. Build a strong knowledge base in your chosen course via the Construction Management Bundle. Benefit from the flexibility and convenience of online learning. With the Construction Management package, validate your learning with a CPD certificate. Each course in this bundle holds a prestigious CPD accreditation, symbolising exceptional quality. The materials, brimming with knowledge, are regularly updated, ensuring their relevance. This bundle promises not just education but an evolving learning experience. Engage with this extraordinary collection, and prepare to enrich your personal and professional development. Embrace the future of learning with the Revit Architecture and Construction Management Fundamentals, a rich anthology of 15 diverse courses. Each course in the Construction Management bundle is handpicked by our experts to ensure a wide spectrum of learning opportunities. This Revit Architecture and Construction Management Fundamentals bundle will take you on a unique and enriching educational journey. The bundle encapsulates our mission to provide quality, accessible education for all. Whether you are just starting your career, looking to switch industries, or hoping to enhance your professional skill set, the Revit Architecture and Construction Management Fundamentals bundle offer you the flexibility and convenience to learn at your own pace. Make the Construction Management package your trusted companion in your lifelong learning journey. CPD 210 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Revit Architecture and Construction Management Fundamentals bundle is perfect for: Lifelong learners looking to expand their knowledge and skills. Professionals seeking to enhance their career with CPD certification. Individuals wanting to explore new fields and disciplines. Anyone who values flexible, self-paced learning from the comfort of home. Career path Unleash your potential with the Revit Architecture and Construction Management Fundamentals bundle. Acquire versatile skills across multiple fields, foster problem-solving abilities, and stay ahead of industry trends. Ideal for those seeking career advancement, a new professional path, or personal growth. Embrace the journey with the Construction Management bundle package. Certificates Certificate Of Completion Digital certificate - Included Certificate Of Completion Hard copy certificate - Included You will get a complimentary Hard Copy Certificate.

SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
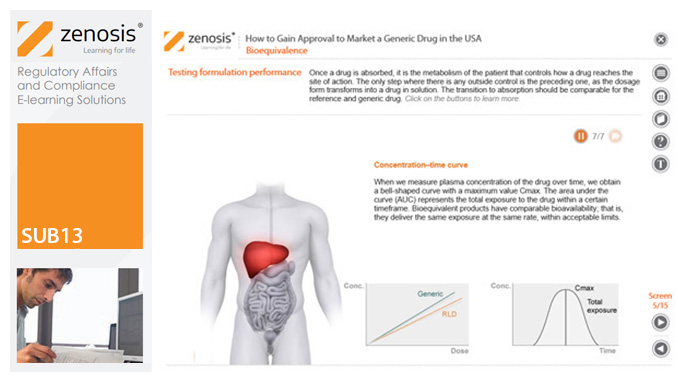
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.