- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11574 Professional Development courses in Colwyn Bay delivered Online
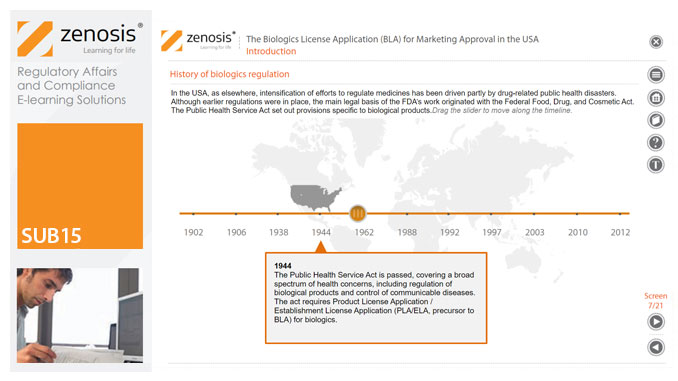
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
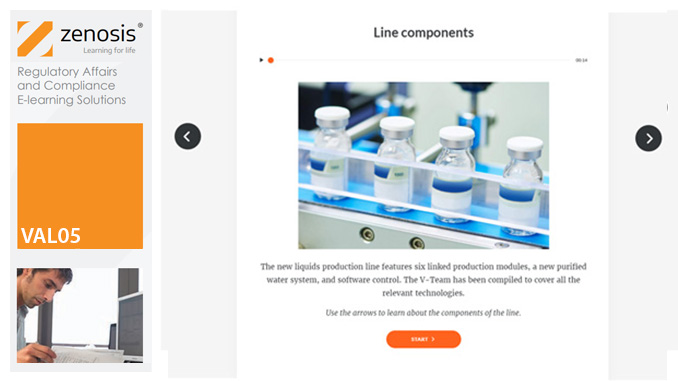
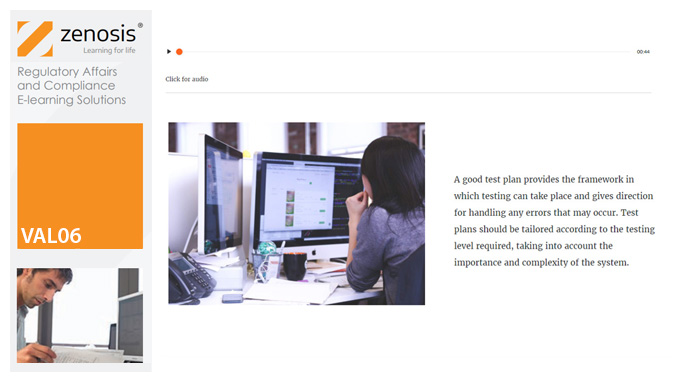
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
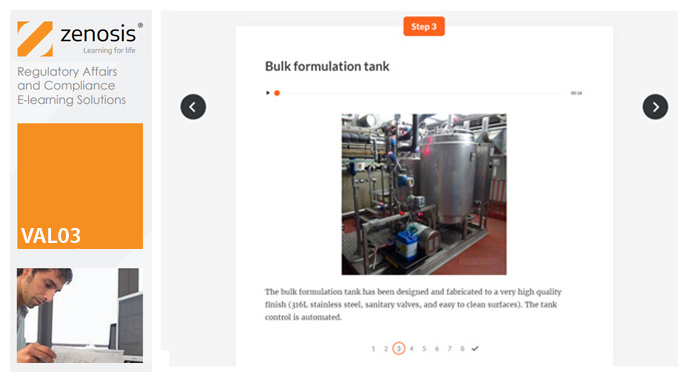
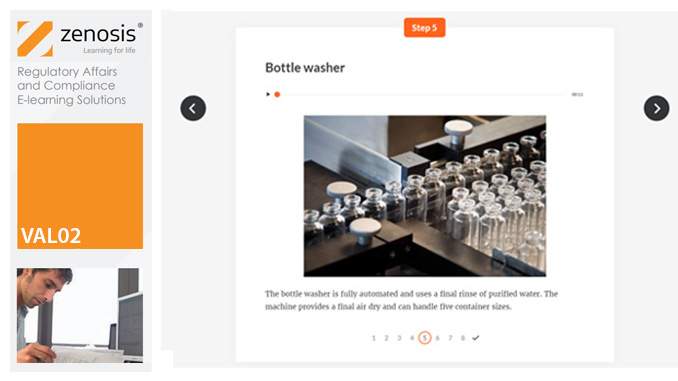
VAL03: Commissioning and Installation Qualification
By Zenosis
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
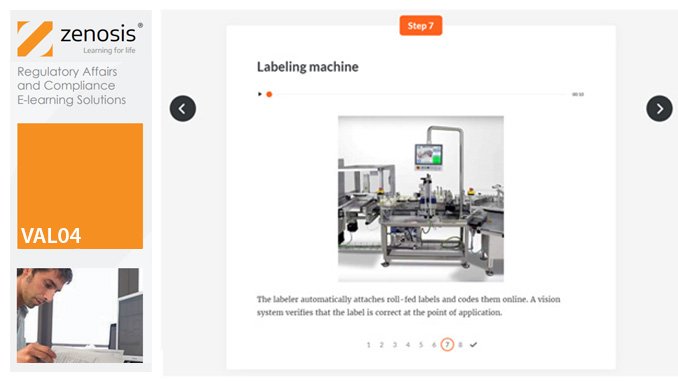
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
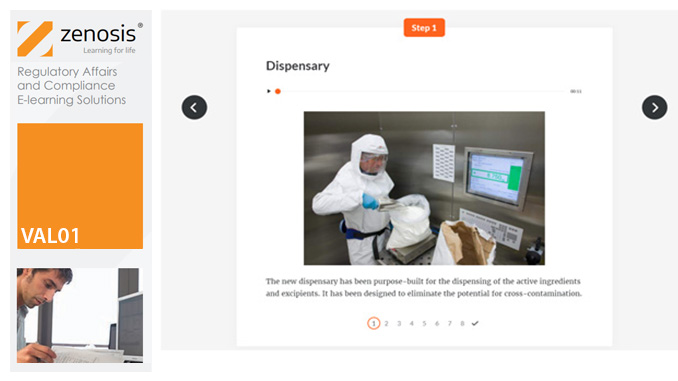
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

Business : Business Starting Up
By Training Tale
With the global financial markets in such turmoil, many people are looking for security in ways they may not have considered previously. This Business Starting Up course is designed for students who want to learn the skills and knowledge required to develop a start-up. It is crucial that you create an effective plan that will allow you to test your product or service and make any necessary changes and improvements. It all starts with developing a successful business idea. This Business Starting Up course comprises several modules that will look into a different aspects of this subject. It will provide learners with an understanding of the initial processes and requirements of a Business Starting Up, as well as knowledge of the first steps in Business Starting Up, including marketing, legal, and financial requirements, as well as an understanding of how to write a start up plan. Learning Outcomes After completing this Business Starting Up course, you will be able to: Understand the steps for a start-up. Understand what is required of you to start your own start up. Polish your start up idea. Build your competitive advantages. Increase self-awareness and aid personal development. Develop a Start-up Business Plan. Why Choose Business START UP Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. Starting Up Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the Starting Up course. Other courses are included with Starting Up Bundle Course Course 01: Business Starting Up Course 02: Level 2 Certificate in Business Management Course 03: Level 2 Diploma in Business Administration Course 04: Level 5 Diploma in Business Analysis Course 05: Level 3 Business Administration Course 06: Level 7 Diploma in Operations Management Course 07: Level 1 Business Management ***Others Included in this Business Starting Up Bundle Course Free 7 PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Free Retake Exam [ Note: Free PDF certificate as soon as completing the course ] Detailed course curriculum of the Starting Up Course: Module 1: Fundamental Steps for a Business Start-up Module 2: Strategic Thinking about New Business Module 3: The Best Business Ideas for You Module 4: Developing a Start-up Business Plan Assessment Method After completing each module of the Business Starting Up, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Business Starting Up course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Business Starting Up course is ideal for anyone who wants to start up his own business. Requirements There are no specific requirements for this Business Starting Up course because it does not require any advanced knowledge or skills. Career path Upon successful completion of this Starting Up course, you may choose to become a: Business Owner Entrepreneur Business Analyst Enterprise Advisor Development Executive Certificates Certificate of completion Digital certificate - Included
