- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
63313 Courses
5 Steps to Unschooling: Rediscovering Play
By LivePlayLearn
You have deregistered your child and you know that unschooling is for you. But, what should you do first? Where do you start? And is it possible to reignite playful times in your child who has had less and less time to actually play since they began going to school? There are lots of things that you can do to move towards unschooling. This webinar concentrates on 5 steps to unschooling that will reconnect your child with their playful selves and provide a solid foundation for future learning. This webinar is FREE for LPL Monthly Members along with a back catalogue of Unschooling Webinars, 2 live webinars a month, and discounts on coaching and mentoring services. Sign up for just £20 per month. Yes! I want to join the LPL Monthly Membership

5 Things to Plan Everyday
By LivePlayLearn
Reporting to the LA when your child is in burnout

Report Writing When Your Child is in Burnout
By LivePlayLearn
Reporting to the LA when your child is in burnout

Couples Therapy: Beyond Foundations - Full Recording
By Practical CBT
Full recording of the workshop: Couples Therapy - Beyond Foundations You will have access to the recording for 14 days.

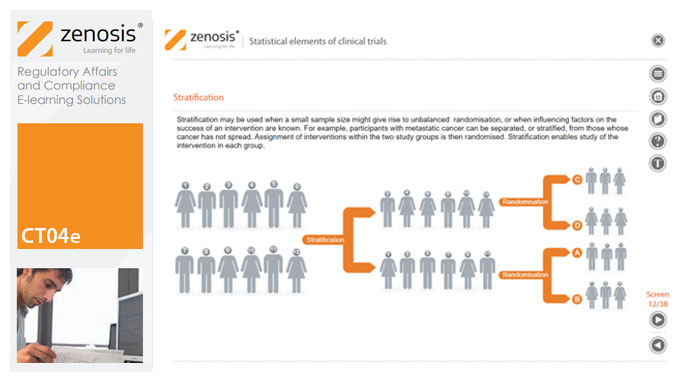
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
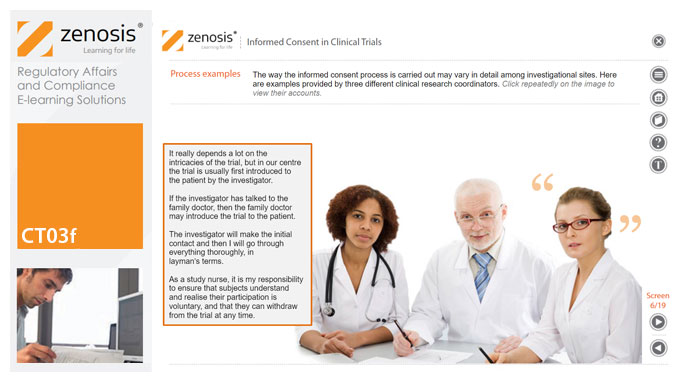
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.
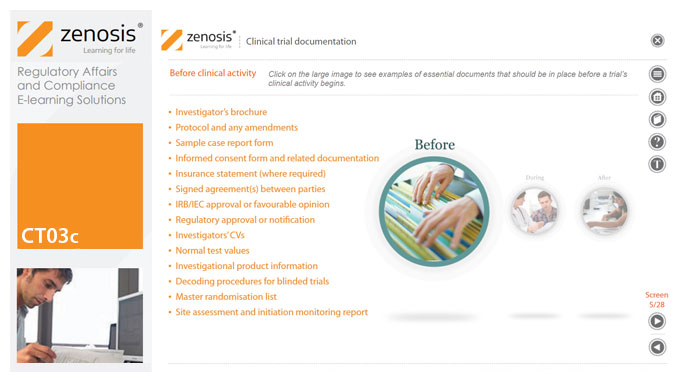
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
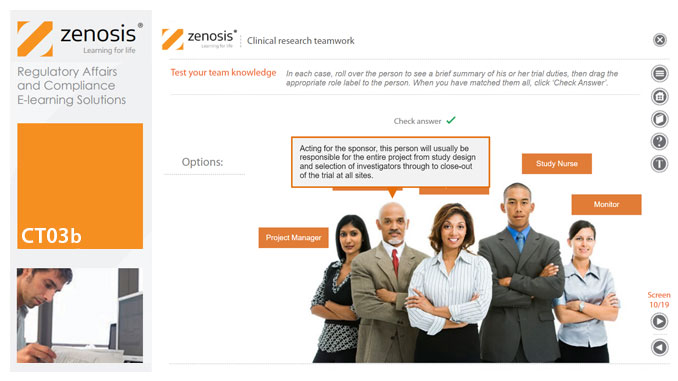
CT03b - Clinical research teamwork
By Zenosis
A clinical trial, particularly a late-phase commercial study, is a major project requiring collaboration between the sponsor and staff or contractor, on the one hand, and the clinical investigator(s) and other healthcare professionals on the other. Good communication among all parties is essential. In this short course we introduce the major roles in a typical clinical research project and outline their duties.
Comorbid GAD and Depression - Full Recording
By Practical CBT
Comorbid GAD and Depression with CBT What is this webinar about? Around 60% of people with anxiety disorders have also have comorbid depression. We also know that people with anxiety disorders and co-occurring depression have higher overall symptom severity and worse outcomes compared to either alone. While training and textbooks focus almost exclusively on protocols for treating anxiety and depression is isolation, the reality in practical clinical settings is that many clients present with both conditions. This practical workshop will examine some key techniques to help clients work on their comorbid anxiety and depression. We will focus on key questions such as which should be treated first? How do we manage psychoeducation? What might be the most useful therapeutic goals in comorbid cases? What are the protocols for transdiagnostic approaches such as the Unified Protocol? Key Topics (indicative) Comorbidty Where to start? Psychoeducation and Comorbidity Common pitfalls to avoid for the therapist working with comorbid conditions The Unified Protocol (Barlow) - practical aspects of implementation Working with Children and Young People with comorbid presentations - the three Pathways (Cummings) Case Study for discussion Summary and Next Steps
Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham