- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
433 Pharmaceutical courses delivered Online
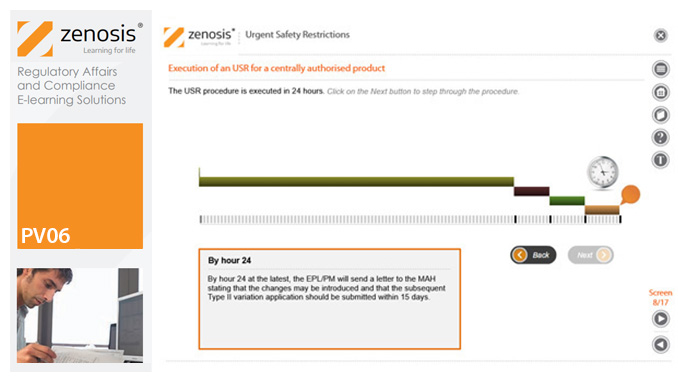
PV06: Urgent Safety Restrictions
By Zenosis
An Urgent Safety Restriction (USR) is a regulatory action taken, in response to a safety signal, to make an interim change to the terms of the marketing authorisation for a medicinal product in Europe. This module describes the principles and procedures for USRs.
Animal Science: 8-in-1 Premium Online Courses Bundle
By Compete High
Dreaming of a career in animal care, veterinary support, or even biomedical research related to animals? 🐶 The Animal Science: 8-in-1 Premium Online Courses Bundle is your gateway into the world of professional pet care, clinical support, and animal-related healthcare. Whether you're aiming to work as a veterinary assistant, in biomedical engineering, or in health & safety roles that relate to animals, this bundle was made to make you hireable. Every course—from dog first aid to nutrition, from animal care to biology—is targeted toward industry needs and job market relevance. Even more important? These aren’t just fancy buzzwords. Animal care, biology, stress management, biomedical engineering, veterinary assistant, nutrition, dog first aid, and health and safety are keywords that hiring platforms like Indeed and LinkedIn actively scan for. So, are you ready to step into a job-ready career path that’s future-proof? 🐾 Let’s go. 📚 Description 🐕 Whether you want to work in vet clinics, rescue shelters, animal research, or pet wellness spaces, the keywords in this bundle are essential to your progression. Start with animal care—the heart of everything. Add in biology and biomedical engineering, and suddenly, you're equipped for technical animal science roles. Combine that with veterinary assistant readiness and you’ve already covered roles in clinics, NGOs, and emergency response centres. Then there’s dog first aid, health and safety, nutrition, and stress management—core topics that signal to employers you can handle animal emergencies, client communication, and daily care. Looking to join a zoo or rescue shelter? They need animal care, nutrition, stress management, and dog first aid skills. Interested in research or pharmaceuticals? You’ll need biology, biomedical engineering, and health and safety credentials. Aiming to freelance in pet wellness or start a grooming service? Knowing dog first aid, veterinary assistant basics, and nutrition makes your brand trustworthy. Whether your end goal is clinic, lab, or kennel—this bundle sets you apart with employer-loved keywords that immediately increase your CV’s value. And with lifetime access and a low one-time cost, you’re saving 80% compared to paying per course. 🐾 ❓ FAQ Q: What roles is this bundle suited for? A: Veterinary support, biomedical lab roles, pet care specialists, dog trainers, animal shelter workers, and freelance consultants in animal care, nutrition, and dog first aid. Q: Do I need prior experience? A: No. All topics—from biology to stress management—are beginner-friendly with clear learning paths. Q: Can I showcase this bundle on my CV? A: Absolutely. Use keywords like veterinary assistant, animal care, dog first aid, and health and safety to boost visibility to hiring managers.

GXP01- Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).

GXP01: Good Practices (GxP) in Drug Development and Manufacturing
By Zenosis
This short entry-level module introduces the learner to good practices (GxP) in drug development and manufacturing. It outlines how the industry operates and how it is regulated. It identifies regulatory authorities and other important sources of guidance on Good Manufacturing Practice (GMP), Good Clinical Practice (GCP), and Good Laboratory Practice (GLP).

Register on the Pharmacology today and build the experience, skills and knowledge you need to enhance your professional development and work towards your dream job. Study this course through online learning and take the first steps towards a long-term career. The course consists of a number of easy to digest, in-depth modules, designed to provide you with a detailed, expert level of knowledge. Learn through a mixture of instructional video lessons and online study materials. Receive online tutor support as you study the course, to ensure you are supported every step of the way. Get a digital certificate as a proof of your course completion. The Pharmacology is incredibly great value and allows you to study at your own pace. Access the course modules from any internet-enabled device, including computers, tablet, and smartphones. The course is designed to increase your employability and equip you with everything you need to be a success. Enrol on the now and start learning instantly! What You Get With The Pharmacology Receive a e-certificate upon successful completion of the course Get taught by experienced, professional instructors Study at a time and pace that suits your learning style Get instant feedback on assessments 24/7 help and advice via email or live chat Get full tutor support on weekdays (Monday to Friday) Course Design The course is delivered through our online learning platform, accessible through any internet-connected device. There are no formal deadlines or teaching schedules, meaning you are free to study the course at your own pace. You are taught through a combination of Video lessons Online study materials Certification Upon successful completion of the course, you will be able to obtain your course completion e-certificate free of cost. Print copy by post is also available at an additional cost of £9.99 and PDF Certificate at £4.99. Who Is This Course For: The course is ideal for those who already work in this sector or are an aspiring professional. This course is designed to enhance your expertise and boost your CV. Learn key skills and gain a professional qualification to prove your newly-acquired knowledge. Requirements: The online training is open to all students and has no formal entry requirements. To study the Pharmacology, all your need is a passion for learning, a good understanding of English, numeracy, and IT skills. You must also be over the age of 16. Course Content Module 01: Fundamental Principles of Pharmacology Fundamental Principles of Pharmacology 00:25:00 Module 02: Drug Development and Regulation Drug Development and Regulation 00:17:00 Module 03: Neuropharmacology Neuropharmacology 00:17:00 Module 04: Cardiovascular Pharmacology Cardiovascular Pharmacology 00:22:00 Module 05: Endocrine Pharmacology Endocrine Pharmacology 00:24:00 Module 06: Chemotherapy Chemotherapy 00:21:00 Module 07: Inflammation and Immune Pharmacology Inflammation and Immune Pharmacology 00:16:00 Module 08: Toxicology Toxicology 00:19:00 Frequently Asked Questions Are there any prerequisites for taking the course? There are no specific prerequisites for this course, nor are there any formal entry requirements. All you need is an internet connection, a good understanding of English and a passion for learning for this course. Can I access the course at any time, or is there a set schedule? You have the flexibility to access the course at any time that suits your schedule. Our courses are self-paced, allowing you to study at your own pace and convenience. How long will I have access to the course? For this course, you will have access to the course materials for 1 year only. This means you can review the content as often as you like within the year, even after you've completed the course. However, if you buy Lifetime Access for the course, you will be able to access the course for a lifetime. Is there a certificate of completion provided after completing the course? Yes, upon successfully completing the course, you will receive a certificate of completion. This certificate can be a valuable addition to your professional portfolio and can be shared on your various social networks. Can I switch courses or get a refund if I'm not satisfied with the course? We want you to have a positive learning experience. If you're not satisfied with the course, you can request a course transfer or refund within 14 days of the initial purchase. How do I track my progress in the course? Our platform provides tracking tools and progress indicators for each course. You can monitor your progress, completed lessons, and assessments through your learner dashboard for the course. What if I have technical issues or difficulties with the course? If you encounter technical issues or content-related difficulties with the course, our support team is available to assist you. You can reach out to them for prompt resolution.

CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
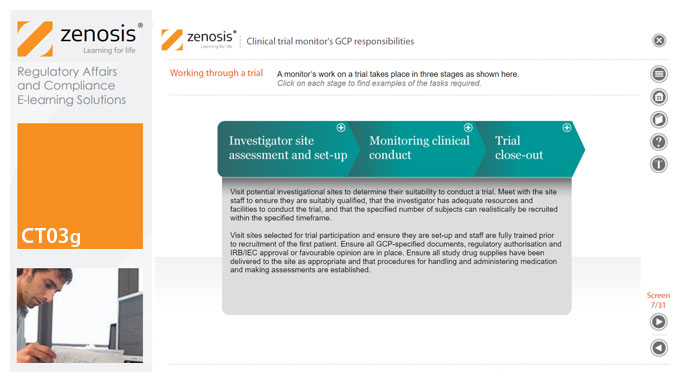
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT03e - Clinical trial investigator’s GCP responsibilities
By Zenosis
A clinical investigator is responsible for conducting the clinical trial in compliance with the study protocol, GCP, medical ethics, and applicable legal requirements. The clinical research community expects that investigators and clinical staff are fully trained in GCP. Duties and functions discussed in this short course include: provision of adequate resources; liaison with IRB/IEC; compliance with protocol; management of investigational product(s), informed consent and data records; and safety reporting.
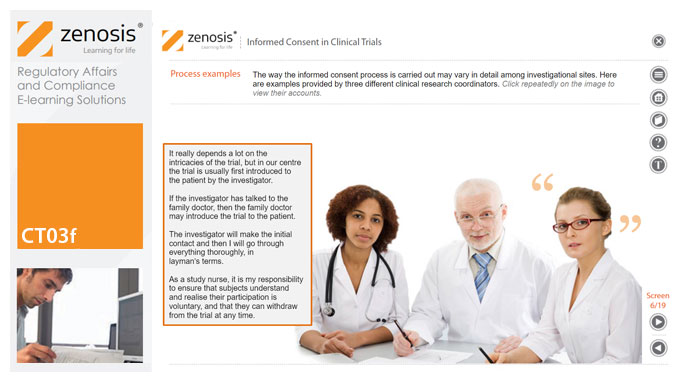
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.