- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
433 Pharmaceutical courses delivered Online
SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

Biomedical Science
By IOMH - Institute of Mental Health
Overview of Biomedical Science Join our Biomedical Science course and discover your hidden skills, setting you on a path to success in this area. Get ready to improve your skills and achieve your biggest goals. The Biomedical Science course has everything you need to get a great start in this sector. Improving and moving forward is key to getting ahead personally. The Biomedical Science course is designed to teach you the important stuff quickly and well, helping you to get off to a great start in the field. So, what are you looking for? Enrol now! This Biomedical Science Course will help you to learn: Learn strategies to boost your workplace efficiency. Hone your skills to help you advance your career. Acquire a comprehensive understanding of various topics and tips. Learn in-demand skills that are in high demand among UK employers This course covers the topic you must know to stand against the tough competition. The future is truly yours to seize with this Biomedical Science. Enrol today and complete the course to achieve a certificate that can change your career forever. Details Perks of Learning with IOMH One-To-One Support from a Dedicated Tutor Throughout Your Course. Study Online - Whenever and Wherever You Want. Instant Digital/ PDF Certificate. 100% Money Back Guarantee. 12 Months Access. Process of Evaluation After studying the course, an MCQ exam or assignment will test your skills and knowledge. You have to get a score of 60% to pass the test and get your certificate. Certificate of Achievement Certificate of Completion - Digital / PDF Certificate After completing the Biomedical Science course, you can order your CPD Accredited Digital / PDF Certificate for £5.99. Certificate of Completion - Hard copy Certificate You can get the CPD Accredited Hard Copy Certificate for £12.99. Shipping Charges: Inside the UK: £3.99 International: £10.99 Who Is This Course for? This Biomedical Science is suitable for anyone aspiring to start a career in relevant field; even if you are new to this and have no prior knowledge, this course is going to be very easy for you to understand. On the other hand, if you are already working in this sector, this course will be a great source of knowledge for you to improve your existing skills and take them to the next level. This course has been developed with maximum flexibility and accessibility, making it ideal for people who don't have the time to devote to traditional education. Requirements You don't need any educational qualification or experience to enrol in the Biomedical Science course. Do note: you must be at least 16 years old to enrol. Any internet-connected device, such as a computer, tablet, or smartphone, can access this online course. Career Path The certification and skills you get from this Biomedical Science Course can help you advance your career and gain expertise in several fields, allowing you to apply for high-paying jobs in related sectors. Course Curriculum Module 01: Introduction to Biomedical Science Introduction to Biomedical Science 00:30:00 Module 02: Genetics and Biochemistry Genetics and Biochemistry 00:19:00 Module 03: Microbiology and Cell Biology Microbiology and Cell Biology 00:26:00 Module 04: Biochemical Engineering and Enzyme Discovery Biochemical Engineering and Enzyme Discovery 00:19:00 Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Toxicology, Pharmaceuticals and Fine Chemicals 00:17:00 Module 06: Systems and Synthetic Biology Systems and Synthetic Biology 00:16:00 Module 07: Global Health Challenges Global Health Challenges 00:12:00 Assignment Assignment - Biomedical Science 00:00:00
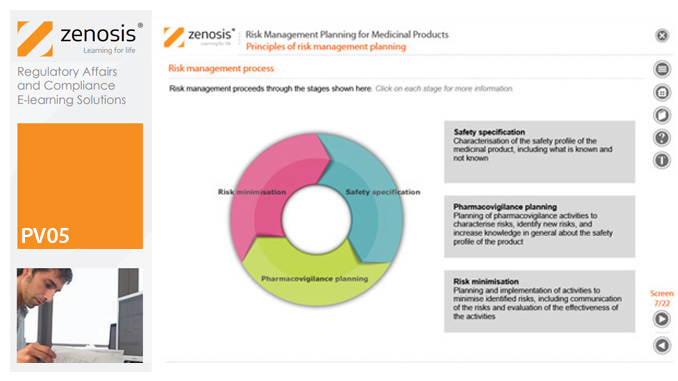
PV05: Risk Management Planning for Medicinal Products
By Zenosis
Proactive risk management is a major component of good pharmacovigilance practice. This module sets out the principles of risk management planning and outlines regulatory requirements for risk management plans in regions that are major markets for medicinal products.
SUB16: The 505(b)(2) Application for Marketing Approval in the USA
By Zenosis
A 505(b)(2) New Drug Application (NDA) is a submission to the Food and Drug Administration (FDA) for approval to market a drug in the USA. It differs from a ‘stand-alone’ NDA in that some of the data on which the applicant relies to demonstrate safety and efficacy have been obtained from publicly available sources rather than from the applicant’s own studies. The applicant typically proposes to market a drug that is based on an approved reference product but modified in its formulation or uses. A 505(b)(2) NDA also differs from an Abbreviated New Drug Application (ANDA) for approval of a generic drug in that the applicant’s product need not be a duplicate of the reference listed drug. The 505(b)(2) pathway may be said to lie part-way between the ‘stand-alone’ NDA and generics pathways, offering a unique combination of advantages to developers. It facilitates the modification of drugs to address unmet medical needs. The 505(b)(2) application pathway accounts for about half of all new drug approvals in the USA.

24 Hours Left! Don't Let the Winter Deals Slip Away - Enrol Now! Gain the required expertise and learn the know-how of Biomedical Science; give a professional look to your portfolio with this highly valued Biomedical Science course Taking your understanding of viruses and global health issues to a new level with our Biomedical Science course. It is perhaps true to say that knowledge of microbiology and virology has never been more important on a worldwide basis than it is now. You can gain specialist tuition in this fascinating field by enrolling with us and completing this Biomedical Science training, helping to work towards a safer future for the planet. This Certificate in Biomedical Science at QLS Level 3 course is endorsed by The Quality Licence Scheme and accredited by CPD (with 120 CPD points) to make your skill development & career progression more accessible than ever! This comprehensive collection of online Biomedical Science modules has been written with the aid of industry experts and contains a total learning curve for the fundamentals of biomedical research. You will learn a wide range of disciplines and theories regarding cellular interactivity and genetics in the modern world. Whether it is for the personal understanding of global diseases and their progression, or for a future career in scientific research, you will find all the answers here for your Biomedical Science studies. Help works towards a disease-free future by taking this Biomedical Science training now. This Biomedical Science training can help you to accomplish your ambitions and prepare you for a meaningful career. So, join us today and gear up for excellence! Key Topics to Be Covered Introduction to biotechnology and toxicology Exploration of microbiology and genetics Working in biochemical engineering and pharmaceuticals Theories of synthetic biology and metabolic engineering Importance of global health challenges Take a step toward a brighter future! Why Prefer This Course? Opportunity to earn a certificate endorsed by the Quality Licence Scheme and another certificate accredited by CPD Get a free student ID card! (£10 postal charges will be applicable for international delivery) Innovative and engaging content. Free assessments 24/7 tutor support. *** Course Curriculum *** Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05: Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Assessment Process You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. After passing the Certificate in Biomedical Science at QLS Level 3 exam, you will be able to a certificate at an additional cost that has been endorsed by the Quality Licence Scheme. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this course. This course is open to everybody. Disclaimer: Kindly be informed that completing this online course does not grant you the authority to practice as a Licensed Biomedical Scientist. If you are searching for a course that fulfils the HCPC's standards, please contact the HCPC or the RCSLT. Requirements You will not need any prior background or expertise to enrol in this course. Career path The Biomedical Science course aims to help you get the job or even the long-awaited promotion of your dreams. With the support and proper guidance, you will learn the skills and knowledge that will help sharpen your career at; Biomedical Technician Biomedical Scientist Biomedical Equipment Technician Biomedical Engineer Certificates CPD Accredited Certificate Digital certificate - £10 Certificate in Biomedical Science at QLS Level 3 Hard copy certificate - £89 After successfully completing the Certificate in Biomedical Science at QLS Level 3, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme. The certificate will be home-delivered, with a pricing scheme of - 89 GBP inside the UK 99 GBP (including postal fees) for international delivery CPD Accredited Certificate 29 GBP for Printed Hardcopy Certificate inside the UK 39 GBP for Printed Hardcopy Certificate outside the UK (international delivery)

Embark on a journey to master the intricacies of drug management with our course, 'Safe and Effective Medication Control and Administration'. This curriculum is meticulously designed to impart profound knowledge and understanding of drug control, ensuring the safety and well-being of patients. The modules cover essential aspects such as legislation, safety, handling, and administration, providing a comprehensive insight into the world of pharmaceuticals. In this course, learners will delve into the nuances of prescribing drugs safely, exploring various types and their appropriate uses. The content is enriched with details on supply, storage, and disposal, ensuring that participants are well-equipped to manage pharmaceuticals effectively. The course also emphasizes the importance of meticulous documentation and record-keeping, a crucial component in drug control. Learning Outcomes Acquire comprehensive knowledge of drug control and administration principles. Understand the legal frameworks and regulations governing pharmaceuticals. Develop proficiency in safe handling, administration, and prescribing. Gain insights into different types of drugs and their appropriate use. Master the skills of accurate documentation and record-keeping. Why buy this Safe and Effective Medication Control and Administration? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards and CIQ after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Unlock career resources for CV improvement, interview readiness, and job success. Who is this Safe and Effective Medication Control and Administration for? Medical practitioners looking to enhance their knowledge in medication management. Pharmacy professionals aiming to understand the broader landscape of medication control. Healthcare administrators seeking to implement best practices in medication processes. Nursing staff aiming to ensure patient safety through effective medication administration. Medical students aspiring to build a strong foundation in medication control and administration. Career path Medical Practitioner: £50,000 - £110,000 Pharmacy Manager: £45,000 - £60,000 Healthcare Administrator: £30,000 - £55,000 Registered Nurse: £23,000 - £35,000 Medication Safety Officer: £40,000 - £60,000 Clinical Documentation Specialist: £35,000 - £50,000 Prerequisites This Safe and Effective Medication Control and Administration does not require you to have any prior qualifications or experience. You can just enrol and start learning. This course was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Module 01: Introduction to Control and Administration of Medication Introduction to Control and Administration of Medication 00:16:00 Module 2: Legislation and Regulations Legislation and Regulations 00:20:00 Module 3: Medication Safety Medication Safety 00:18:00 Module 4: Safe Handling of Medication Safe Handling of Medication 00:18:00 Module 5: Safe Administration of Medication Safe Administration of Medication 00:15:00 Module 6: Prescribing Medication Safely Prescribing Medication Safely 00:16:00 Module 7: Different Types of Medication Different Types of Medication 00:18:00 Module 8: Supply, Storage and Disposal Supply, Storage and Disposal 00:15:00 Module 9: Documentation and Record Keeping Documentation and Record Keeping 00:17:00

Overview This comprehensive course on Biomedical Science will deepen your understanding on this topic. After successful completion of this course you can acquire the required skills in this sector. This Biomedical Science comes with accredited certification which will enhance your CV and make you worthy in the job market. So enrol in this course today to fast track your career ladder. How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is this course for? There is no experience or previous qualifications required for enrolment on this Biomedical Science. It is available to all students, of all academic backgrounds. Requirements Our Biomedical Science is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible on tablets and smartphones so you can access your course on wifi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career path Having these various qualifications will increase the value in your CV and open you up to multiple sectors such as Business & Management, Admin, Accountancy & Finance, Secretarial & PA, Teaching & Mentoring etc. Course Curriculum 8 sections • 8 lectures • 02:19:00 total length •Introduction to Biomedical Science: 00:30:00 •Genetics and Biochemistry: 00:19:00 •Microbiology and Cell Biology: 00:26:00 •Biochemical Engineering and Enzyme Discovery: 00:19:00 •Toxicology, Pharmaceuticals and Fine Chemicals: 00:17:00 •Systems and Synthetic Biology: 00:16:00 •Global Health Challenges: 00:12:00 •Assignment - Biomedical Science: 00:00:00

Dive deep into the fascinating realm of Biomedical Science with this Biomedical Science at QLS Level 3 course, a discipline that amalgamates both biology and medicine, striving to understand and conquer the myriad challenges humanity faces. With a curriculum spanning genetics, biochemistry, global health challenges, and more, this course offers a comprehensive immersion into the world of cutting-edge biomedical studies. Whether you're aiming to decipher the genetic codes that make us who we are or keen to uncover solutions to pressing global health crises, this course will guide you through the scientific marvels and mysteries of our bodies and the wider world. Learning Outcomes: Understand the fundamental concepts and principles of Biomedical Science. Gain insights into the intricacies of genetics, biochemistry, and cell biology. Develop a knowledge base in biochemical engineering, enzyme discovery, and toxicology. Analyse the role and implications of pharmaceuticals and fine chemicals in health. Explore the challenges and solutions in global health from a biomedical perspective. Why choose this Biomedical Science at QLS Level 3 course? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments are designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Who is this Biomedical Science at QLS Level 3 course for? Aspiring biomedical researchers keen on delving into the molecular and cellular aspects of health and disease. Graduates in life sciences aiming to extend their knowledge in specialised biomedical disciplines. Health professionals seeking a deeper understanding of the biological foundations underpinning their practice. Students with a profound interest in global health and the challenges encountered on a worldwide scale. Anyone with a penchant for science and an eagerness to discover how biomedical knowledge can reshape our future. Career path Biomedical Scientist: £25,000 - £45,000 Annually Genetic Counsellor: £28,000 - £51,000 Annually Microbiologist: £23,000 - £50,000 Annually Biochemical Engineer: £30,000 - £60,000 Annually Toxicologist: £22,000 - £48,000 Annually Systems Biologist: £27,000 - £55,000 Annually Prerequisites This Biomedical Science at QLS Level 3 does not require you to have any prior qualifications or experience. You can just enrol and start learning.This course was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Endorsed Certificate of Achievement from the Quality Licence Scheme Learners will be able to achieve an endorsed certificate after completing the course as proof of their achievement. You can order the endorsed certificate for only £85 to be delivered to your home by post. For international students, there is an additional postage charge of £10. Endorsement The Quality Licence Scheme (QLS) has endorsed this course for its high-quality, non-regulated provision and training programmes. The QLS is a UK-based organisation that sets standards for non-regulated training and learning. This endorsement means that the course has been reviewed and approved by the QLS and meets the highest quality standards. Please Note: Studyhub is a Compliance Central approved resale partner for Quality Licence Scheme Endorsed courses. Course Curriculum Module 01: Introduction to Biomedical Science Introduction to Biomedical Science 00:30:00 Module 02: Genetics and Biochemistry Genetics and Biochemistry 00:19:00 Module 03: Microbiology and Cell Biology Microbiology and Cell Biology 00:26:00 Module 04: Biochemical Engineering and Enzyme Discovery Biochemical Engineering and Enzyme Discovery 00:19:00 Module 05:Toxicology, Pharmaceuticals and Fine Chemicals Toxicology, Pharmaceuticals and Fine Chemicals 00:17:00 Module 06: Systems and Synthetic Biology Systems and Synthetic Biology 00:16:00 Module 07: Global Health Challenges Global Health Challenges 00:12:00 Assignment Assignment - Biomedical Science at QLS Level 3 02:00:00 Order your QLS Endorsed Certificate Order your QLS Endorsed Certificate 00:00:00

CT12: How to Conduct Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course describes the requirements that must be met by, and options available to, the sponsor during the conduct of an authorised clinical trial. It identifies the various interactions with MSCs that occur via the Clinical Trials Information System (CTIS), and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT11 sets out the European legal and regulatory context for clinical trials and describes how to apply via the CTIS for authorisation to conduct trials. The two courses therefore provide an ideal foundation for understanding and complying with the new law.

MD01: An Introduction to the Regulation of Medical Devices
By Zenosis
This module provides an introduction to the basics of medical device regulation, especially the requirements that manufacturers must meet in order to market devices in Europe and the USA.
