- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
402 Pharmaceutical courses delivered Online
Pharmacy Technician & Pharmacy Assistant - Training
By Training Tale
Pharmacy Technician & Pharmacy Assistant This Pharmacy Technician & Pharmacy Assistant course is customised only for you! Do you want to advance your career in the healthcare industry? Or, are you looking for a way to contribute to community health care without performing clinical duties? If you're considering a career in the pharmaceutical industry, then you've come to the right place. This Pharmacy Technician & Pharmacy Assistant course teaches you everything you need to know to become a Pharmacy Technician or Pharmacy Technician Assistant and provide support and stability to your team. By enrolling on our Pharmacy Technician & Pharmacy Assistant course, you will become familiar with the roles and responsibilities of a Pharmacy Assistant or Technician. This comprehensive course will teach you the fundamental techniques of a Pharmacy worker and how to successfully counsel and diagnose a patient. You will also gain experience in the practice of prescribing and dispensing medication, as well as stock control and inventory management. Along with this, you will gain knowledge of medical health and safety standards and security protocols associated with legal drugs. Enrol in this Pharmacy Technician & Pharmacy Assistant course now and start your career in the UK's healthcare sector. Courses are included in this Pharmacy Technician & Pharmacy Assistant Bundle Course Course 01: Pharmacy Assistant & Technician Course 02: Level 2 Certificate in Understanding Nutrition and Health Course 03: Level 5 Mental Health First Aid Certification Other Benefits of this Bundle Course Free 3 PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Free Retake Exam [ Note: Free PDF certificate will provide as soon as completing the Pharmacy Technician & Pharmacy Assistant course] Pharmacy Technician & Pharmacy Assistant Course Curriculum of Pharmacy Technician & Pharmacy Assistant This Pharmacy Technician & Pharmacy Assistant course consists of 09 modules. Assessment Method After completing each module of the Pharmacy Technician & Pharmacy Assistant course, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Pharmacy Technician & Pharmacy Assistant course, you will be entitled to a Certificate of Completion from Training Tale. Who is this course for? Pharmacy Technician & Pharmacy Assistant This Pharmacy Technician & Pharmacy Assistant course is ideal for those interested in becoming pharmacy technicians or those looking to make a career in the medical field. Requirements Pharmacy Technician & Pharmacy Assistant There are no specific requirements for this Pharmacy Technician & Pharmacy Assistant course because it does not require any advanced knowledge or skills. Career path Pharmacy Technician & Pharmacy Assistant Certificates Certificate of completion Digital certificate - Included

This Diploma in Pharmacology at QLS Level 4 course is endorsed by The Quality Licence Scheme and accredited by CPDQS (with 120 CPD points) to make your skill development & career progression more accessible than ever! Are you familiar with Alexander Fleming's discovery of penicillin in 1928? That was the birth of modern pharmacology, which has since saved millions of lives worldwide. If you want to contribute to the ever-evolving field of medicine, then our Pharmacology Training course is the perfect opportunity for you. In this course, you will learn about the fundamental principles of pharmacology, drug development and regulation, neuropharmacology, chemotherapy, and more. Our comprehensive eight-module course covers a wide range of topics, including inflammation and immune pharmacology, and toxicology. You will have the opportunity to develop a deep understanding of pharmacology and learn how to apply your knowledge to real-world scenarios. This Pharmacology course will guide you through each module, ensuring that you have a strong grasp of each topic before moving on to the next one. By the end of this course, you will have a thorough understanding of pharmacology and be able to apply your knowledge to real-world scenarios. You will also develop the skills necessary to evaluate and analyse pharmacological data, understand drug development and regulation, and be able to work in teams to solve complex problems. This course will equip you with the knowledge and skills necessary to succeed in the field of pharmacology. After this Pharmacology Training course, you will be able to learn: Understand the fundamental principles of pharmacology Analyze drug development and regulation Evaluate the effects of neuropharmacology on the body Understand cardiovascular and endocrine pharmacology Analyze chemotherapy treatments for various diseases Understand inflammation and immune pharmacology Why Prefer This Pharmacology at QLS Level 5 Diplloma from HF Online? Pharmacology FREE certificate endorsed by the Quality Licence Scheme & another accredited by CPDQS Get a free student ID card! (£10 postal charge will be applicable for international delivery) with Pharmacology course Innovative and engaging content of Pharmacology Free assessments available with Pharmacology 24/7 tutor support available Get Lifetime Access Get Free CPD Certified PDF Certificate Take a step toward a brighter future! *** Course Curriculum of Pharmacology at QLS Level 4*** Module 01: Fundamental Principles of Pharmacology Module 02: Drug Development and Regulation Module 03: Neuropharmacology Module 04: Cardiovascular Pharmacology Module 05: Endocrine Pharmacology Module 06: Chemotherapy Module 07: Inflammation and Immune Pharmacologyc Module 08: Toxicology Assessment Process of Pharmacology at QLS Level 4 You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you have completed all of the Diploma in Pharmacology at QLS Level 4 exams. CPD 120 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring pharmacologists Medical students Pharmaceutical researchers Healthcare professionals Individuals interested in the field of medicine Science graduates Requirements No prior background or expertise is required to enrol on this course. Career path Pharmacologist Pharmaceutical Scientist Medical Science Liaison Clinical Research Associate Pharmacovigilance Officer Regulatory Affairs Manager Certificates CPDQS Accredited Certificate Digital certificate - Included Diploma in Pharmacology at QLS Level 4 Hard copy certificate - Included Show off Your New Skills with a Certificate of Completion After successfully completing the Diploma in Pharmacology at QLS Level 4, you can order an original hardcopy certificate of achievement endorsed by the Quality Licence Scheme andalso you can order CPDQSAccredited Certificate that is recognised all over the UK and also internationally. The certificates will be home-delivered, completely free of cost.

Diploma in Pharmacy Skills
By The Teachers Training
Diploma in Pharmacy Skills is yet another 'Teacher's Choice' course from Teachers Training for a complete understanding of the fundamental topics. You are also entitled to exclusive tutor support and a professional CPD-accredited certificate in addition to the special discounted price for a limited time. Just like all our courses, this Diploma in Pharmacy Skills and its curriculum have also been designed by expert teachers so that teachers of tomorrow can learn from the best and equip themselves with all the necessary skills. Consisting of several modules, the course teaches you everything you need to succeed in this profession. The course can be studied part-time. You can become accredited within 12 hour studying at your own pace. Your qualification will be recognised and can be checked for validity on our dedicated website. Why Choose Teachers Training Some of our website features are: This is a dedicated website for teaching 24/7 tutor support Interactive Content Affordable price Courses accredited by the UK's top awarding bodies 100% online Flexible deadline Entry Requirements No formal entry requirements. You need to have: Passion for learning A good understanding of the English language Be motivated and hard-working Over the age of 16. Certification CPD Certification from The Teachers Training Successfully completing the MCQ exam of this course qualifies you for a CPD-accredited certificate from The Teachers Training. You will be eligible for both PDF copy and hard copy of the certificate to showcase your achievement however you wish. You can get your digital certificate (PDF) for £4.99 only Hard copy certificates are also available, and you can get one for only £10.99 You can get both PDF and Hard copy certificates for just £12.99! The certificate will add significant weight to your CV and will give you a competitive advantage when applying for jobs. Diploma in Pharmacy Skills Module 01 : Introduction to Pharmacy 00:25:00 Module 02 : History and Development of Pharmacy 00:27:00 Module 03 : The Pharmacy Team and Practices 00:17:00 Module 04 : Training of the Pharmacy Team 00:42:00 Module 05 : Coaching and Evaluation of the Pharmacy Team 00:18:00 Module 06 : Standards for Pharmacy Professionals 00:34:00 Module 07 : Guidance to Support the Standards for Pharmacy Professionals 01:41:00 Module 08 : The Rights and Responsibilities of Pharmacy Professionals 01:06:00 Module 09 : Pharmacy Practice 00:55:00 Module 10 : Clinical Pharmacy Skills 01:19:00 Module 11 : Offering Enhanced Services 01:08:00 Module 12 : Prescribing 00:59:00 Module 13 : Supplying Medication 00:48:00 Module 14 : Responding to Symptoms 00:44:00 Module 15 : Health and Safety in the Pharmaceutical Industry 00:42:00

[vc_row][vc_column][vc_column_text] Description Want to work in pharmaceuticals? A pharmacy assistant works in a variety of settings, from retail stores to hospitals, supporting licensed pharmacists through clerical work, receiving prescriptions and general administrative tasks. If you're considering a career in pharmaceuticals, this online Pharmacy Assistant Diploma will give you the practical skills and knowledge you need to succeed in this industry. Throughout this pharmacy assistant course, you will learn how to perform essential pharmacy staff administrative duties, including the role of the pharmacist's assistant and how to provide excellent customer service to meet your customers' prescription needs. Besides the basics, you'll learn to perform various tasks related to drug inventory, such as taking orders, packaging and dispensing medicines, preparing prescription labels, and so on. Operating the register, handling money transactions, and performing clerical work will also be explained in this introductory course. If you are interested in becoming a pharmacy assistant or dispensing assistant within a healthcare team, this training program will help you accelerate your professional development and give you the training you need to become successful in this field. No previous qualifications or entry requirements are needed to take this course. Assessment: This course does not involve any MCQ test. Students need to answer assignment questions to complete the course, the answers will be in the form of written work in pdf or word. Students can write the answers in their own time. Once the answers are submitted, the instructor will check and assess the work. Certification: After completing and passing the course successfully, you will be able to obtain an Accredited Certificate of Achievement. Certificates can be obtained either in hard copy at a cost of £39 or in PDF format at a cost of £24. Who is this Course for? Pharmacy Assistant Diploma is certified by CPD Qualifications Standards and CiQ. This makes it perfect for anyone trying to learn potential professional skills. As there is no experience and qualification required for this course, it is available for all students from any academic background. Requirements Our Pharmacy Assistant Diploma is fully compatible with any kind of device. Whether you are using Windows computer, Mac, smartphones or tablets, you will get the same experience while learning. Besides that, you will be able to access the course with any kind of internet connection from anywhere at any time without any kind of limitation. Career Path After completing this course you will be able to build up accurate knowledge and skills with proper confidence to enrich yourself and brighten up your career in the relevant job market.[/vc_column_text][/vc_column][/vc_row] Pharmacy Assistant Diploma Module 1: Introduction to Pharmacy Assistant and Pharmacy Technician 00:16:00 Module 2: Job Role of Pharmacy Technicians 00:25:00 Module 3: Pharmacy Assistant Patient Counselling Guide 00:20:00 Module 4: Communication in Pharmacy Settings 00:16:00 Module 5: The Pharmacy Team and Practices 00:17:00 Module 6: Prescription and Dispensing in Pharmacies 00:20:00 Module 7: Dispensing Methods, EPS, Minimising Dispensing Errors in Pharmacies 00:17:00 Module 8: Inventory Control and Management in Pharmacies 00:20:00 Module 9: Standard Operating Procedures (SOPs) 00:13:00 Module 10: Health and Safety Risks Assessment and Pharmaceutical Terminology 00:21:00 Order Your Certificates and Transcripts Order Your Certificates and Transcripts 00:00:00

Level 3 Certificate in Biomedical Science
By Compliance Central
This Level 3 Certificate in Biomedical Science will take you on an exciting journey into the core of science and discovery. Imagine yourself in the vanguard of invention, solving the cellular puzzles of life and discovering the keys to optimal human wellness. It's more than simply a course; Level 3 Certificate in Biomedical Science is a doorway to a world where every discovery can transform people's lives and influence medical research in the future. Dive head first into the realms of Biomedical Science, where each module is a portal to new knowledge and understanding. From delving into the intricacies of genetics and biochemistry to exploring the wonders of microbiology and cell biology, every step of this journey is an exhilarating adventure into the unknown. With modules spanning from biochemical engineering to global health challenges, Level 3 Certificate in Biomedical Science course equips you with the tools and insights needed to make groundbreaking contributions to the field. Prepare to be captivated by the wonders of science, as you uncover the complexities of life itself. Join us on this extraordinary journey, and let your curiosity lead the way to a future filled with endless possibilities in Biomedical Science. Level 3 Certificate in Biomedical Science course Learning Outcomes: Understand the fundamentals of Biomedical Science and its applications. Explore the intricacies of genetics, biochemistry, and microbiology. Gain insights into biochemical engineering and enzyme discovery. Delve into toxicology, pharmaceuticals, and fine chemicals. Discover the principles of systems and synthetic biology. Address global health challenges through the lens of Biomedical Science. Level 3 Certificate in Biomedical Science Module 01: Introduction to Biomedical Science Module 02: Genetics and Biochemistry Module 03: Microbiology and Cell Biology Module 04: Biochemical Engineering and Enzyme Discovery Module 05: Toxicology, Pharmaceuticals and Fine Chemicals Module 06: Systems and Synthetic Biology Module 07: Global Health Challenges Certificate of Achievement Endorsed Certificate of Achievement from the Quality Licence Scheme Learners will be able to achieve an endorsed certificate after completing the Level 3 Certificate in Biomedical Science course as proof of their achievement. You can order the endorsed certificate for Free to be delivered to your home by post. For international students, there is an additional postage charge of £10. Endorsement The Quality Licence Scheme (QLS) has endorsed this Level 3 Certificate in Biomedical Science course for its high-quality, non-regulated provision and training programmes. The QLS is a UK-based organisation that sets standards for non-regulated training and learning. This endorsement means that the Level 3 Certificate in Biomedical Science course has been reviewed and approved by the QLS and meets the highest quality standards. Who is this course for? Level 3 Certificate in Biomedical Science Who is this course for: Aspiring Biomedical Scientists Healthcare Professionals Medical Researchers Pharmaceutical Technicians Laboratory Technologists Requirements Level 3 Certificate in Biomedical Science To enrol in this Level 3 Certificate in Data Protection (GDPR) Practitioner course, all you need is a basic understanding of the English Language and an internet connection. Career path Level 3 Certificate in Biomedical Science Career Paths: Welcome to a realm of boundless opportunities! Explore diverse career paths after completing this Level 3 Certificate in Biomedical Science course. Embrace the journey ahead in Biomedical Science. Biomedical Scientist Clinical Research Associate Pharmaceutical Analyst Laboratory Manager Genetics Counsellor Certificates CPD Accredited PDF Certificate Digital certificate - Included QLS Endorsed Hard Copy Certificate Hard copy certificate - Included CPD Accredited Hard Copy Certificate Hard copy certificate - £9.99 CPD Accredited Hard Copy Certificate Delivery Charge: Inside the UK: Free Outside of the UK: £9.99 each

Dive into the dynamic realm of clinical data analysis with our comprehensive Clinical Data Analysis with SAS course. This course is your passport to the pharmaceutical industry, guiding you through the essential components, phases of clinical trials, and types of data crucial in this field. You'll gain proficiency in interpreting clinical study documents, from protocols to ethical consent, enabling you to navigate the intricate world of clinical data. Our course equips you with SAS programming skills, empowering you to develop clinical study reports, analyze demographic data, and derive valuable insights. Whether you're a budding data analyst or a professional aiming to enhance your clinical data expertise, this course provides the knowledge and skills needed for a successful career in clinical data analysis. Learning Outcomes Understand the key components and phases of the pharmaceutical industry. Navigate clinical trials with insights into data types and reports. Interpret clinical study documents, including protocols and ethical consent. Develop clinical study reports using SAS programming. Analyze demographic data and derive valuable insights. Why choose this Clinical Data Analysis with SAS course? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards and CIQ after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Unlock career resources for CV improvement, interview readiness, and job success. Who is this Clinical Data Analysis with SAS course for? Aspiring clinical data analysts seeking to enter the pharmaceutical industry. Professionals in healthcare, research, or data analysis looking to enhance their clinical data expertise. Students and individuals interested in clinical data and its analysis. Those who want to decode clinical study documents and reports. Anyone aiming to unlock the world of clinical data analysis with SAS. Career path Clinical Data Analyst: £25,000 - £50,000 Biostatistician: £30,000 - £70,000 Pharmaceutical Researcher: £25,000 - £60,000 Data Scientist in Healthcare: £30,000 - £70,000 Clinical SAS Programmer: £28,000 - £60,000 Clinical Research Manager: £35,000 - £80,000 Prerequisites This Clinical Data Analysis with SAS does not require you to have any prior qualifications or experience. You can just enrol and start learning.This Clinical Data Analysis with SAS was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Course Promo Course Promo 00:01:00 Section 01: Introduction 1.1 Components of the Pharma Industry 00:05:00 1.2 Phases of Clinical Trials 00:06:00 1.3 Data and Reports in Clinical Trials 00:04:00 1.4 Types of Data 00:05:00 Section 02: Knowledge on Clinical Study Documents 2.1 Clinical Study Protocol 00:02:00 2.2 Ethical Consent 00:01:00 2.3 Inclusion-Exclusion Criteria 00:01:00 2.4 Statistical Analysis Plan: SAP, Mockshell and CRF 00:04:00 Section 03: Developing the Clinical Study Reports 3.1 General SAS Programming Steps 00:02:00 3.2 One Search Report: Demographics Table 00:04:00 3.3 Understanding the Demographics Table 00:03:00 3.4 Programming the Demographics Table 00:05:00 3.5 Importing Raw Demographic Data into the SAS 00:04:00 3.6 Deciding what Procedure to Use 00:02:00 3.7 Deriving the AGE variable 00:10:00 3.8 Obtaining Summary Statistics for AGE 00:04:00 3.9 Adding the 3rd Treatment Group using Explicit Output 00:05:00 3.10 Deriving the SEX variable 00:03:00 3.11 Obtaining Summary Statistics for SEX 00:03:00 3.12 Concatenating the COUNT and PERCENT Variables 00:03:00 3.13 Deriving the RACE Variable 00:03:00 3.14 Obtaining Summary Statistics for RACE 00:03:00 3.15 Stacking All the 3 Summary Statistics Together 00:06:00 3.16 Fixing the Precision Points 00:04:00 3.17 Transposing Data 00:03:00 3.18 Fixing the Order of Statistical Parameters 00:05:00 3.19 Building the Final Report 00:02:00 3.20 Putting the Final Touches to the Report 00:11:00 Resources Resources - Clinical Data Analysis with SAS 00:00:00 Assignment Assignment - Clinical Data Analysis with SAS 00:00:00

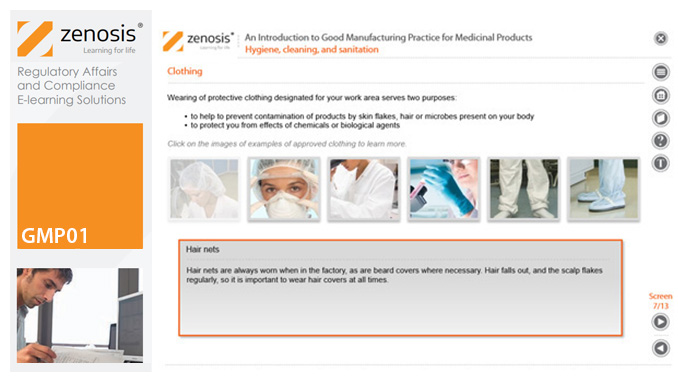
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
Project Management and Construction Management at QLS Level 7
By Imperial Academy
Level 7 - Two Endorsed Diploma | QLS Hard Copy Certificate Included | Plus 5 CPD Courses | Lifetime Access

Good Clinical Practices: A Practical Guide to GCP Compliance
By Xpert Learning
About Course Understand the Ethical and Regulatory Framework for Conducting Clinical Trials Course Description This comprehensive Good Clinical Practices (GCP) course provides a thorough understanding of the ethical and regulatory principles governing clinical trials. It delves into the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines, ensuring you grasp the essential standards for conducting clinical research. Course Objectives By the end of this course, you will be able to: Articulate the definition, purpose, and historical context of GCP. Explain the importance of GCP in protecting human subjects and ensuring data integrity. Identify key international organizations involved in establishing GCP standards. Apply ethical principles and informed consent procedures in clinical research. Understand the ICH E6(R2) guidelines on Integrated Addendum to Good Clinical Practice. Design and conduct clinical trials according to ICH E8 and E9 guidelines. Manage and report clinical trial data in compliance with ICH E6(R2) guidelines. Implement safety monitoring and adverse event reporting procedures as per ICH E6(R2) and E6(R3) guidelines. Navigate regulatory compliance and inspections related to clinical trials. Target Audience This course is designed for individuals who: Are interested in pursuing a career in clinical research or clinical trial management. Work in the pharmaceutical, biotechnology, or medical device industry. Seek to gain a comprehensive understanding of GCP principles and practices. Prerequisites No prior experience in clinical research is required. However, a basic understanding of research methodology and medical terminology is recommended. Please Note: This course is strictly theoretical and does not qualify participants for clinical practice in the industry. Additional training and certifications may be required for hands-on experience. What Will You Learn? Articulate the definition, purpose, and historical context of GCP. Explain the importance of GCP in protecting human subjects and ensuring data integrity. Identify key international organizations involved in establishing GCP standards. Apply ethical principles and informed consent procedures in clinical research. Understand the ICH E6(R2) guidelines on Integrated Addendum to Good Clinical Practice. Design and conduct clinical trials according to ICH E8 and E9 guidelines. Manage and report clinical trial data in compliance with ICH E6(R2) guidelines. Implement safety monitoring and adverse event reporting procedures as per ICH E6(R2) and E6(R3) guidelines. Navigate regulatory compliance and inspections related to clinical trials. Course Content Introduction to Good Clinical Practice (GCP) and ICH Guidelines Introduction to Good Clinical Practice (GCP) and ICH Guidelines Ethical Principles, Informed Consent, and ICH E6(R2) Ethical Principles, Informed Consent, and ICH E6(R2) Designing and Conducting Clinical Trials with ICH E8 and E9 Designing and Conducting Clinical Trials with ICH E8 and E9 Data Management and Reporting with ICH E6(R2) Data Management and Reporting with ICH E6(R2) Safety and Monitoring in Clinical Trials with ICH E6(R2) and E6(R3) Safety and Monitoring in Clinical Trials with ICH E6(R2) and E6(R3) Regulatory Compliance, Inspections, and ICH E6(R3) Regulatory Compliance, Inspections, and ICH E6(R3) A course by Xpert Learning RequirementsA basic understanding of research methodology and medical terminology is recommended. Audience Individuals who are interested in pursuing a career in clinical research or clinical trial management. Individuals who work in the pharmaceutical, biotechnology, or medical device industry. Individuals who seek to gain a comprehensive understanding of GCP principles and practices. Audience Individuals who are interested in pursuing a career in clinical research or clinical trial management. Individuals who work in the pharmaceutical, biotechnology, or medical device industry. Individuals who seek to gain a comprehensive understanding of GCP principles and practices.

