- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Effecting Business Process Improvement: In-House Training
By IIL Europe Ltd
Effecting Business Process Improvement: In-House Training Business analysts facilitate the solution of business problems. The solutions are put into practice as changes to the way people perform in their organizations and the tools they use. The business analyst is a change agent who must understand the basic principles of quality management. This course covers the key role that business analysts play in organizational change management. What you will Learn You will learn how to: Define and document a business process Work with various business modeling techniques Perform an enterprise analysis in preparation for determining requirements Analyze business processes to discern problems Foundation Concepts Overview of business analysis and process improvement Defining the business process Introducing the proactive business analyst Focusing on business process improvement for business analysts Launching a Successful Business Process Improvement Project Overview of the launch phase Understanding and creating organizational strategy Selecting the target process Aligning the business process improvement project's goals and objectives with organizational strategy Defining the Current Process Overview of current process phase Documenting the business process Business modeling options: work-flow models Business modeling options: Unified Modeling Language (UML) model adaptations for business processes Analyzing the Current Process Process analysis overview Evaluation: establishing the control group Opportunity techniques: multi-discipline problem-solving Opportunity techniques: matrices Building and Sustaining a Recommended Process Overview of the recommended process and beyond Impact analysis Recommended process Transition to the business case Return to proactive state

Effecting Business Process Improvement
By IIL Europe Ltd
Effecting Business Process Improvement Business analysts facilitate the solution of business problems. The solutions are put into practice as changes to the way people perform in their organizations and the tools they use. The business analyst is a change agent who must understand the basic principles of quality management. This course covers the key role that business analysts play in organizational change management. What you will Learn You will learn how to: Define and document a business process Work with various business modeling techniques Perform an enterprise analysis in preparation for determining requirements Analyze business processes to discern problems Foundation Concepts Overview of business analysis and process improvement Defining the business process Introducing the proactive business analyst Focusing on business process improvement for business analysts Launching a Successful Business Process Improvement Project Overview of the launch phase Understanding and creating organizational strategy Selecting the target process Aligning the business process improvement project's goals and objectives with organizational strategy Defining the Current Process Overview of current process phase Documenting the business process Business modeling options: work-flow models Business modeling options: Unified Modeling Language (UML) model adaptations for business processes Analyzing the Current Process Process analysis overview Evaluation: establishing the control group Opportunity techniques: multi-discipline problem-solving Opportunity techniques: matrices Building and Sustaining a Recommended Process Overview of the recommended process and beyond Impact analysis Recommended process Transition to the business case Return to proactive state

Risk Management for IT Projects
By IIL Europe Ltd
Risk Management for IT Projects IT projects may have direct bottom-line impact on the organization, cost millions of dollars, cause organizational change and change the way the organization is perceived by clients. Many IT projects are notoriously hard to predict and are filled with risk. IT Risk Management takes a comprehensive look at IT project risk management using PMI's PMBOK® Guide Risk Management Model in the context of IT Project Life Cycle phases. The goal of this course is to arm the practitioner with a rigorous, common-sense approach to addressing uncertainty in projects. This approach includes the ability to influence project outcomes, avoid many potential project risks, and be ready to calmly and efficiently respond to unavoidable challenges. What you will Learn You'll learn how to: Describe the risk management process, using the PMBOK® Guide's standard models and terminology Discuss the potential barriers to managing risk effectively in IT project organizations Develop an effective risk management plan for IT projects Identify project risks using IT-specific, practical tools Analyze individual risk events and overall project risk using IT-specific, practical approaches Plan effective responses to IT-specific risk based on the results of risk analysis and integrate risk responses into project schedules and cost estimates Manage and control risk throughout the IT project life cycle Implement selected elements of IT project risk management on your next project Foundation Concepts Basic concepts and purpose Risk and project constraints Risk and corporate cultures Risk management and IT PLC standards Plan Risk Management for IT Projects Plan Risk management process Plan Risk management activities Design a standard template Assess the project-specific needs Tailor the template Produce a project-specific risk management plan Gain consensus and submit as part of overall project plan A risk management plan of IT projects Identify Risks for IT Projects Identify risk process overview Risk categories and examples Risk identification tools Risk events by project life-cycle phases Perform Risk Analysis for IT Projects Perform qualitative risk analysis overview Core qualitative tools for IT projects Auxiliary qualitative tools for cost and schedule estimates When to use quantitative analysis for IT projects Plan Risk Response for IT Projects Plan risk response overview Active risk response strategies for IT projects (Threat and Opportunity) Acceptance and contingency reserves Contingency planning for IT projects Plan risk responses for IT projects Implement Risk Response for IT Projects Implement Risk Responses Executing Risk Response Plans Techniques and Tools Used Continuous Risk Management Monitor Risks for IT Projects Monitor risks overview Monitor risks tips for IT projects Technical performance measurement systems Risk management implementation for IT projects

Risk Management for IT Projects: In-House Training
By IIL Europe Ltd
Risk Management for IT Projects: In-House Training IT projects may have direct bottom-line impact on the organization, cost millions of dollars, cause organizational change and change the way the organization is perceived by clients. Many IT projects are notoriously hard to predict and are filled with risk. IT Risk Management takes a comprehensive look at IT project risk management using PMI's PMBOK® Guide Risk Management Model in the context of IT Project Life Cycle phases. The goal of this course is to arm the practitioner with a rigorous, common-sense approach to addressing uncertainty in projects. This approach includes the ability to influence project outcomes, avoid many potential project risks, and be ready to calmly and efficiently respond to unavoidable challenges. What you will Learn You'll learn how to: Describe the risk management process, using the PMBOK® Guide's standard models and terminology Discuss the potential barriers to managing risk effectively in IT project organizations Develop an effective risk management plan for IT projects Identify project risks using IT-specific, practical tools Analyze individual risk events and overall project risk using IT-specific, practical approaches Plan effective responses to IT-specific risk based on the results of risk analysis and integrate risk responses into project schedules and cost estimates Manage and control risk throughout the IT project life cycle Implement selected elements of IT project risk management on your next project Foundation Concepts Basic concepts and purpose Risk and project constraints Risk and corporate cultures Risk management and IT PLC standards Plan Risk Management for IT Projects Plan Risk management process Plan Risk management activities Design a standard template Assess the project-specific needs Tailor the template Produce a project-specific risk management plan Gain consensus and submit as part of overall project plan A risk management plan of IT projects Identify Risks for IT Projects Identify risk process overview Risk categories and examples Risk identification tools Risk events by project life-cycle phases Perform Risk Analysis for IT Projects Perform qualitative risk analysis overview Core qualitative tools for IT projects Auxiliary qualitative tools for cost and schedule estimates When to use quantitative analysis for IT projects Plan Risk Response for IT Projects Plan risk response overview Active risk response strategies for IT projects (Threat and Opportunity) Acceptance and contingency reserves Contingency planning for IT projects Plan risk responses for IT projects Implement Risk Response for IT Projects Implement Risk Responses Executing Risk Response Plans Techniques and Tools Used Continuous Risk Management Monitor Risks for IT Projects Monitor risks overview Monitor risks tips for IT projects Technical performance measurement systems Risk management implementation for IT projects

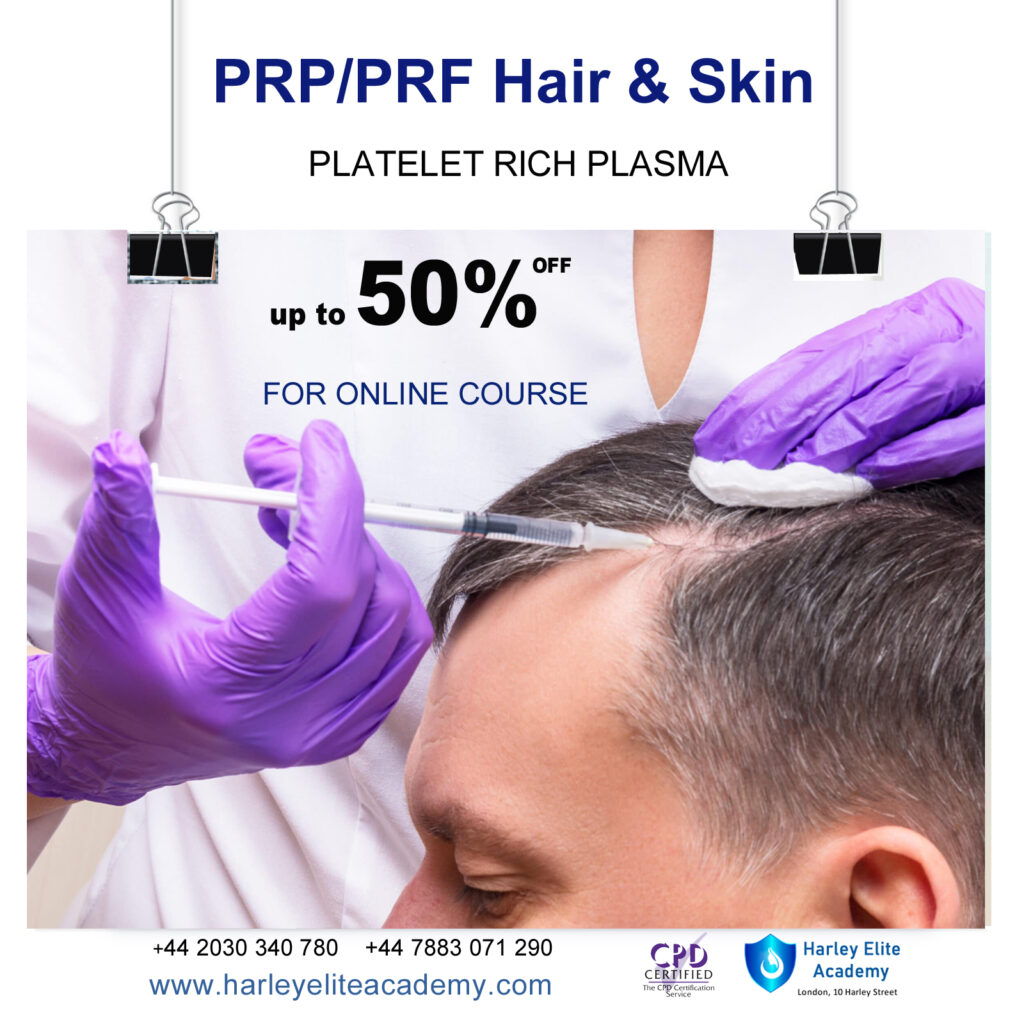
PRP / PRF SKIN & HAIR COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! CLINICAL PRP • Sports medicine • Traumatology • Ophthalmic • Burn trauma • Wound healing –diabetic foot • Skin grafting • Dentistry-sinus lift • Tooth implants. PRP theory & equipment: Training Online Theory will enable you to understand: Anatomy Vascular Supply, Contraindications Patient consultation Complications Management Post treatment advice Dealing with equipment A certification of training will be provided upon completion of the course. Aesthetic PRP • Skin rejuvenation • Hair restoration • Fat grafting in combination PRP • Post laser •Acne & Rosacea •Acne scar •TissueVolumisation alternative of HA fillers •Aesthetic gynecology /urology. Plathelet Rich Plasma We will cover pertinent information including mechanism of action, safety and efficacy issues, management and treatment of complications, dilution guidelines, and more. Hands on practical session – skin rejuvenation and hair loss Extraction, Preparation and Dosage Management Injection techniques – face, neck and head (hair loss); also the use of cannula Upon successful completion of the course, you will receive a certificate and title of PRP Certified Practitioner. MASTER CLASS PRP & PRF During the course we are providing . Taking blood and how to use a Centrifuge . PRP injecting techniques in face neck and décolletage hands. PRP Microneedling using a DERMAPEN. Combination treatment PRP with Mesotherapy. MECHANISM OF ACTION Platelets + Leucocytes form 3D mesh release of GF Chemo attraction and migration of macrophages and stem cells Stem cells proliferates by mitosis Stem cells undergo differentiation process BENEFIT FROM PRP TREATMENT & THERAPYExperience the advantages of PRP treatment and therapy, utilizing autologous blood with natural growth factors for disease-free and hypoallergenic benefits. Boost wound healing by regulating mitosis, proliferation, and differentiation, enhancing tissue with collagen, elastin, and hyaluronic acid. Benefit from improved tissue oxygenation, nutrition flow, and support for procedures like hair transplants, fat transfers, and skin grafts.PRP works effectively in skin rejuvenation, facial resurfacing, microneedling, and combines well with HA, PDO threads, skin boosters, peeling, or CO2 lasers. It also proves beneficial for hair restoration, showing positive results in various protocols for Androgenic alopecia and age-related hair loss.PRP where works .Skin rejuvenation-facial resurfacing.application-injection alone. Microneedling Combination with HA,Combination with PDO threads,Skin boosters , peeling or CO2 lasers Hair restoration, Multiple protocols with positive results Evidence for improvement of: Androgenic alopecia-male and females, “spot hair lost” Improvement of age related hair loss. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
LNG Terminal Operations & Safety
By EnergyEdge - Training for a Sustainable Energy Future
Elevate your expertise in LNG terminal operations safety through our classroom training course. Energyedge provides industry-leading expertise and guidance.

Promoting Best Practice in Asthma and Anaphylaxis Training (Train the Trainer)
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in asthma and anaphylaxis management with our evidence-based training course. Ideal for healthcare professionals, educators, and patient care providers. Optimise patient outcomes with effective emergency response protocols, prevention strategies, and patient education.
Well Integrity (Basic and Advanced)
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This intensive 5 full-day has been designed as a separately bookable course comprising 3 days of Well Integrity (Basic) and 2 days of Advanced Well Integrity. The intensive 3 full-day course will equip the participants with a thorough knowledge of well integrity management and risk assessment in producing assets. Based on the regulatory requirements and using real examples and exercises from around the world, this represents best practice integrity management within the oil and gas industry. When to take action with a well is a critical decision, both from a safety and economic perspective. A consistent approach to decision-making provides certainty within the organisation, focusing effort, and spending wisely. The decision-making steps will be set out to ensure all critical aspects are captured consistently. Risk analysis approaches used by different organisations and examples of risk management and risk-ranking methods will be discussed. The 2 full-day course will deepen the participants' knowledge of well integrity management, and skills for designing, operating, and maintaining well equipment. The ultimate goal is to optimise productivity at the lowest Unit Operating Cost (UOC) and to maintain mechanical integrity throughout well life cycle. Well Integrity management is looked at in three distinct stages. The first stage is during the well design which includes material selection, engineering design, cement design, coating and inhibitors and cathodic protection. The second stage is monitoring the well during the life of the well, locating possible leaks and / or loss of metal. The last stage is to manage and control any well integrity issues using specialised products, services and techniques. Training Objectives 1. Well Integrity Training: Upon completion of this course, the participants will be able to: Define the building blocks of a successful well integrity management system Develop an approach to risk management, understand risk analysis and methods applied across the industry How do we 'Make Wells Safer', learn about emerging technologies for well integrity problem diagnosis and new techniques available to 'repair' the issues Execute the basic elements of well integrity management training for field operators Evaluate well design elements that enhance or hinder well integrity status definition during the operating phase of the well life-cycle Gain the demonstrable benefits of well integrity management from field experience Review cases studies and discuss them to enhance knowledge and take on board lessons learned 2. Advanced Well Integrity: Define well integrity well categorization based on compliance to the barrier policy outlined in the regulations and develop an approach to risk management Discuss well-completion design and construction to create a 'integer' well with the lowest life cycle maintenance cost from a WIM perspective Monitoring and surveillance of well integrity, focusing on barrier competence such as cementing and corrosion Investigate and manage well integrity issues, causes & potential solutions Understand repairs needed to address 'Loss of Well Operating Envelope' Gain an overview understanding of Well Suspension & Well Abandonment Discuss further case studies as well as conduct a post course test Target Audience Invaluable for production, operations, and integrity professionals involved in implementing & managing well integrity and seeking to improve performance. It is also essential for those who need to develop and implement such systems, or who have a general need to know and understand more about well integrity management. The course will also provide a fresh approach for senior professionals and managers. Designed for professionals in the oil and gas industry who are involved in the design, construction and operation of wells from the following disciplines: Production Maintenance Production Operations Drilling Engineering Safety engineering Well Intervention Well Integrity Engineering Asset Management Course Level Intermediate Advanced Trainer Gordon Duncan has over 40 years of experience in the Oil & Gas industry. During that time, he has worked exclusively in well intervention and completions. After a number of years working for intervention service companies (completions, slickline & workovers), he joined Shell as a well service supervisor. He was responsible for the day-to-day supervision of all well intervention work on Shell's Persian/Arabian Gulf platforms. This included completion running, coil tubing, e-line, slickline, hydraulic workovers, well testing and stimulation operations. An office-based role as a senior well engineer followed. He was responsible for planning, programming and organising of all the well engineering and intervention work on a number of fields in the Middle East. He had a brief spell as a Site Representative for Santos in Australia before joining Petro-Canada as Completions Superintendent in Syria, then moved to Australia as Completions Operations Superintendent for Santos, before returning to Shell as Field Supervisor Completions and Well Interventions in Iraq where he carried out the first ever formal abandonment of a well in the Majnoon Field. While working on rotation, he regularly taught Completion Practices, Well Intervention, Well Integrity and Reporting & Planning courses all over the world. In 2014, he started to focus 100% on training and became the Technical Director for PetroEDGE. Since commencing delivering training courses in 2008, he has taught over 300 courses in 31 cities in 16 countries to in excess of 3,500 participants. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

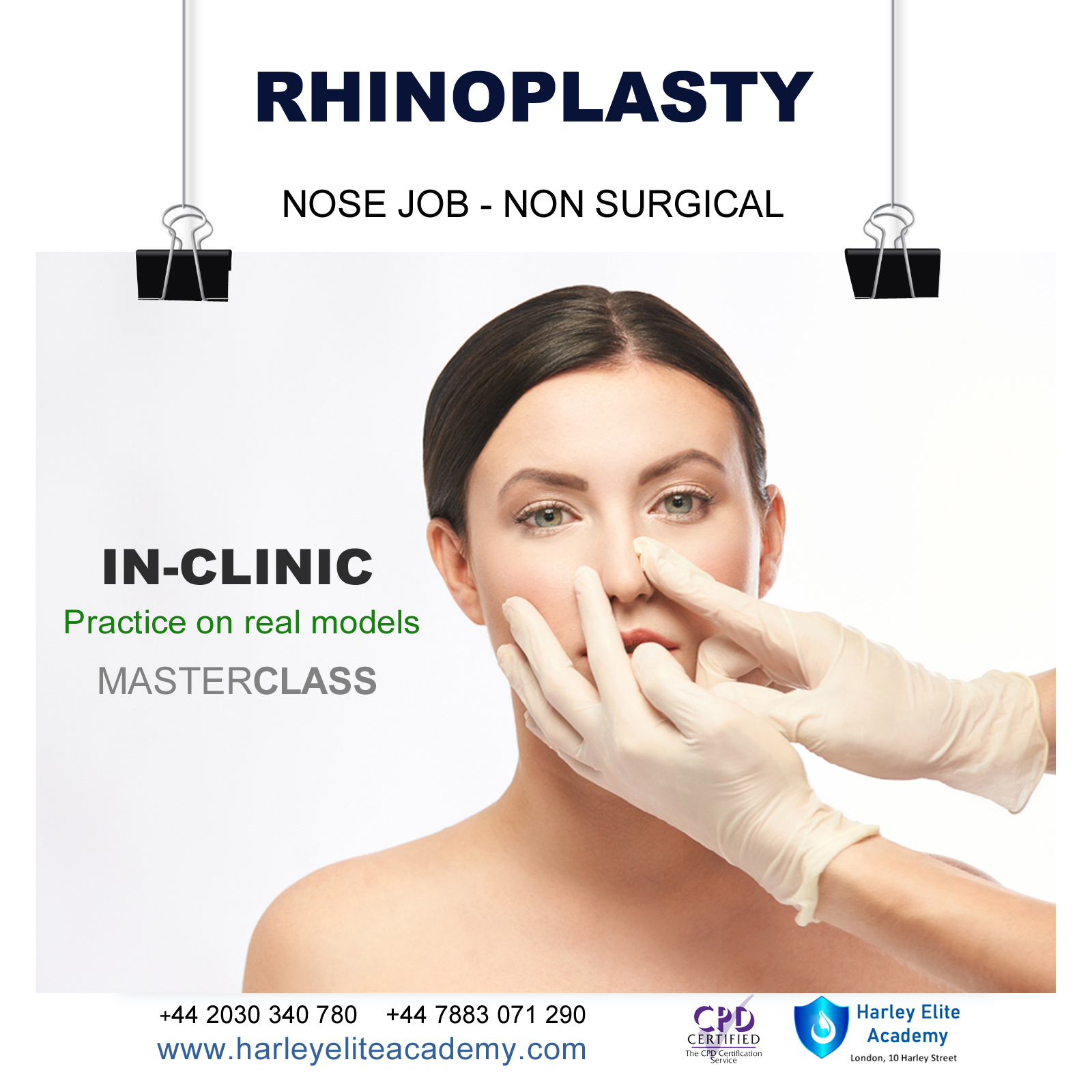
RHINOPLASTY NOSE JOB COURSE
By Harley Elite Academy (HeLa)
MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! On this course, we aim to help you master a technique that will set you apart from most routine cosmetic treatment providers and enable you to step into the future of advanced cosmetic. Training THEORY will enable you to understand: Anatomy Vascular Supply, Nerves on the face Contraindications Patient consultation ONE-TO-ONE Training Nose Job Masterclass You will perform this procedure on live models under the supervision and guidance of highly experienced aesthetic practitioners. You will be trained under ENT specialist. We will give you all the knowledge you need for a safe technique in your practice. A certification of training will be provided upon completion of the course. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (Theory), IN CLINIC (Practice) COURSE LEVEL EXPERT | Masterclass Course
Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Search By Location
- Pha Courses in London
- Pha Courses in Birmingham
- Pha Courses in Glasgow
- Pha Courses in Liverpool
- Pha Courses in Bristol
- Pha Courses in Manchester
- Pha Courses in Sheffield
- Pha Courses in Leeds
- Pha Courses in Edinburgh
- Pha Courses in Leicester
- Pha Courses in Coventry
- Pha Courses in Bradford
- Pha Courses in Cardiff
- Pha Courses in Belfast
- Pha Courses in Nottingham