- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8628 Nursing courses
Health & Safety in a Care Setting Course Online
By Lead Academy
This Course at a Glance Understand that a Care home is both a home for vulnerable residents and a work environment Understand the duties and responsibilities of both the employer and employee in maintaining health and safety Explore the most common legislations that apply to care homes Understand how to design care plans to ensure the highest level of care involved Understand how to assist a person with first aid in emergency situations Learn how to carry out a proper risk assessment to identify risks and potential hazards Know when and how to report health and safety risks Identify the various types of illnesses and accidents that can occur within care settings Recognise the risks and hazards that can arise from moving and handling Acquire knowledge about the 6Cs of nursing Know how to deal with general hazards arising from fire, gas and electricity Gain knowledge about a range of effective solutions to deal with work-related stress, violence and aggression Know how to implement proper security measures to prevent individuals from becoming victims of crimes Gain knowledge about the potential hazards in the working environment and the range of welfare requirements that apply to care workers Health & Safety in a Care Setting Course Overview This extensive Health and Safety in Care Setting course is designed to help you identify the common hazards and risks found within care homes and provides concise insight on how to prevent such risks at an early stage. You will also gain knowledge about certain legislations that apply to care homes, the responsibilities of management and employees in ensuring the health and safety of vulnerable residents and outlines the procedure of conducting effective risk assessments within premises. Adverse occurrences as a result of unsafe health care are one of the top ten causes of death and disability worldwide. It is estimated that approximately one out of every ten patients in high-income countries is likely to be in the position of being harmed while obtaining hospital care. A wide range of adverse poor health and safety protocols can cause such harm, with about half of them being preventable. Therefore, it is extremely important for care homes to set up effective health and safety protocols to prevent such harm and ensure the safety of the vulnerable people residing in the care setting. Care homes are not always workplaces but also home to a number of vulnerable residents. These residents as well the people working in the care settings have the right to reside and work in a safe and healthy environment that respects their rights, freedoms, and dignity. Many potential health and safety problems exist in a care home setting, and they must be identified and analysed in order to be effectively handled. Upon successful completion of this Health and Safety in Care Setting course, you will be able to detect the wide range of dangers that can be present in care homes that may arise during care and treatment and prevent and mitigate such mishaps through proper risk assessments and procedures. Who should take this course? Whether you are an employer or an employee working in any care home setting or anyone simply looking to escalate your knowledge on mental health awareness to assist yourself or people around you exposed to unsafe environments in any care settings, this Health and Safety in Care Setting course is ideal for you. Entry Requirements There are no academic entry requirements for this Health & Safety in a Care Setting Course Online course, and it is open to students of all academic backgrounds. However, you are required to have a laptop/desktop/tablet or smartphone and a good internet connection. Assessment Method This Health and Safety in Care Setting Course assesses learners through multiple choice questions (MCQs). Upon successful completion of each module, learners must answer MCQs to step into the next module. Through the MCQs it is measured how much a learner could grasp from each section. In the assessment pass mark is 75%. Course Curriculum Module 1: Introduction Module 2: Responsibilities Module 3: Systems Module 4: Accidents and Illness Part 1 Module 5: Accidents and Illness Part 2 Module 6: Moving and Handling Module 7: General Hazards: Fires, Gas and Electricity Module 8: Aggression, Violence, Security and Stress Module 9: Work Environment and Welfare Recognised Accreditation CPD Certification Service This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. CPD certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Many organisations look for employees with CPD requirements, which means, that by doing this course, you would be a potential candidate in your respective field. Certificate of Achievement Valuable Certification Upon successful completion of this Health and Safety in Care Setting Course, you will be eligible to download CPD accredited free electronic certificate instantly. There is a minimal shipping charge applicable to get the hardcopy course completion certificate which is: Shipment Inside the UK = £5.99 International Shipment = £16.99 Overview This Course at a Glance Understand that a Care home is both a home for vulnerable residents and a work environment Understand the duties and responsibilities of both the employer and employee in maintaining health and safety Explore the most common legislations that apply to care homes Understand how to design care plans to ensure the highest level of care involved Understand how to assist a person with first aid in emergency situations Learn how to carry out a proper risk assessment to identify risks and potential hazards Know when and how to report health and safety risks Identify the various types of illnesses and accidents that can occur within care settings Recognise the risks and hazards that can arise from moving and handling Acquire knowledge about the 6Cs of nursing Know how to deal with general hazards arising from fire, gas and electricity Gain knowledge about a range of effective solutions to deal with work-related stress, violence and aggression Know how to implement proper security measures to prevent individuals from becoming victims of crimes Gain knowledge about the potential hazards in the working environment and the range of welfare requirements that apply to care workers Health & Safety in a Care Setting Course Overview This extensive Health and Safety in Care Setting course is designed to help you identify the common hazards and risks found within care homes and provides concise insight on how to prevent such risks at an early stage. You will also gain knowledge about certain legislations that apply to care homes, the responsibilities of management and employees in ensuring the health and safety of vulnerable residents and outlines the procedure of conducting effective risk assessments within premises. Adverse occurrences as a result of unsafe health care are one of the top ten causes of death and disability worldwide. It is estimated that approximately one out of every ten patients in high-income countries is likely to be in the position of being harmed while obtaining hospital care. A wide range of adverse poor health and safety protocols can cause such harm, with about half of them being preventable. Therefore, it is extremely important for care homes to set up effective health and safety protocols to prevent such harm and ensure the safety of the vulnerable people residing in the care setting. Care homes are not always workplaces but also home to a number of vulnerable residents. These residents as well the people working in the care settings have the right to reside and work in a safe and healthy environment that respects their rights, freedoms, and dignity. Many potential health and safety problems exist in a care home setting, and they must be identified and analysed in order to be effectively handled. Upon successful completion of this Health and Safety in Care Setting course, you will be able to detect the wide range of dangers that can be present in care homes that may arise during care and treatment and prevent and mitigate such mishaps through proper risk assessments and procedures. Who should take this course? Whether you are an employer or an employee working in any care home setting or anyone simply looking to escalate your knowledge on mental health awareness to assist yourself or people around you exposed to unsafe environments in any care settings, this Health and Safety in Care Setting course is ideal for you. Entry Requirements There are no academic entry requirements for this Health & Safety in a Care Setting Course Online course, and it is open to students of all academic backgrounds. However, you are required to have a laptop/desktop/tablet or smartphone and a good internet connection. Assessment Method This Health and Safety in Care Setting Course assesses learners through multiple choice questions (MCQs). Upon successful completion of each module, learners must answer MCQs to step into the next module. Through the MCQs it is measured how much a learner could grasp from each section. In the assessment pass mark is 75%. Course Curriculum Module 1: Introduction Module 2: Responsibilities Module 3: Systems Module 4: Accidents and Illness Part 1 Module 5: Accidents and Illness Part 2 Module 6: Moving and Handling Module 7: General Hazards: Fires, Gas and Electricity Module 8: Aggression, Violence, Security and Stress Module 9: Work Environment and Welfare Recognised Accreditation CPD Certification Service This course is accredited by continuing professional development (CPD). CPD UK is globally recognised by employers, professional organisations, and academic institutions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. CPD certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Many organisations look for employees with CPD requirements, which means, that by doing this course, you would be a potential candidate in your respective field. Certificate of Achievement Valuable Certification Upon successful completion of this Health and Safety in Care Setting Course, you will be eligible to download CPD accredited free electronic certificate instantly. There is a minimal shipping charge applicable to get the hardcopy course completion certificate which is: Shipment Inside the UK = £5.99 International Shipment = £16.99 FAQ Is CPD a recognised qualification in the UK? CPD is globally recognised by employers, professional organisations and academic intuitions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. CPD-certified certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Are QLS courses recognised? Although QLS courses are not subject to Ofqual regulation, they must adhere to an extremely high level that is set and regulated independently across the globe. A course that has been approved by the Quality Licence Scheme simply indicates that it has been examined and evaluated in terms of quality and fulfils the predetermined quality standards. When will I receive my certificate? For CPD accredited PDF certificate it will take 24 hours, however for the hardcopy CPD certificate takes 5-7 business days and for the Quality License Scheme certificate it will take 7-9 business days. Can I pay by invoice? Yes, you can pay via Invoice or Purchase Order, please contact us at info@lead-academy.org for invoice payment. Can I pay via instalment? Yes, you can pay via instalments at checkout. How to take online classes from home? Our platform provides easy and comfortable access for all learners; all you need is a stable internet connection and a device such as a laptop, desktop PC, tablet, or mobile phone. The learning site is accessible 24/7, allowing you to take the course at your own pace while relaxing in the privacy of your home or workplace. Does age matter in online learning? No, there is no age limit for online learning. Online learning is accessible to people of all ages and requires no age-specific criteria to pursue a course of interest. As opposed to degrees pursued at university, online courses are designed to break the barriers of age limitation that aim to limit the learner's ability to learn new things, diversify their skills, and expand their horizons. When I will get the login details for my course? After successfully purchasing the course, you will receive an email within 24 hours with the login details of your course. Kindly check your inbox, junk or spam folder, or you can contact our client success team via info@lead-academy.org Course Fee £25 £60 58% OFF ð CPD UK Accredited PDF Certificate Included 4 interest-free payments of £6.25 Health & Safety in a Care Setting Course Online quantity ENROL NOW Duration: * Hours Instant Access Get In Touch Today Live CHAT WITH US CALL ON - 0300 124 5585 Certificate of Achievement Valuable Certification Upon successful completion of this Health and Safety in Care Setting Course, you will be eligible to download CPD accredited free electronic certificate instantly. There is a minimal shipping charge applicable to get the hardcopy course completion certificate which is: Shipment Inside the UK = £5.99 International Shipment = £16.99 FAQs Is CPD a recognised qualification in the UK? CPD is globally recognised by employers, professional organisations and academic intuitions, thus a certificate from CPD Certification Service creates value towards your professional goal and achievement. CPD-certified certificates are accepted by thousands of professional bodies and government regulators here in the UK and around the world. Are QLS courses recognised? Although QLS courses are not subject to Ofqual regulation, they must adhere to an extremely high level that is set and regulated independently across the globe. A course that has been approved by the Quality Licence Scheme simply indicates that it has been examined and evaluated in terms of quality and fulfils the predetermined quality standards. When will I receive my certificate? For CPD accredited PDF certificate it will take 24 hours, however for the hardcopy CPD certificate takes 5-7 business days and for the Quality License Scheme certificate it will take 7-9 business days. Can I pay by invoice? Yes, you can pay via Invoice or Purchase Order, please contact us at info@lead-academy.org for invoice payment. Can I pay via instalment? Yes, you can pay via instalments at checkout. How to take online classes from home? Our platform provides easy and comfortable access for all learners; all you need is a stable internet connection and a device such as a laptop, desktop PC, tablet, or mobile phone. The learning site is accessible 24/7, allowing you to take the course at your own pace while relaxing in the privacy of your home or workplace. Does age matter in online learning? No, there is no age limit for online learning. Online learning is accessible to people of all ages and requires no age-specific criteria to pursue a course of interest. As opposed to degrees pursued at university, online courses are designed to break the barriers of age limitation that aim to limit the learner's ability to learn new things, diversify their skills, and expand their horizons. When I will get the login details for my course? After successfully purchasing the course, you will receive an email within 24 hours with the login details of your course. Kindly check your inbox, junk or spam folder, or you can contact our client success team via info@lead-academy.org

SENCO : Special Educational Needs Coordination
By Training Tale
This SENCO : Special Educational Needs Coordination is designed for those interested in or who are currently in a SENCO role in the early years setting. This SENCO : Special Educational Needs Coordination qualification provides learners with a thorough understanding of the roles and responsibilities of the Special Educational Needs Coordinator in early years setting. Learn about the strategies and techniques for assisting children and their families and gain in-depth knowledge of SEN codes of practice. The purpose of this is to help learners progress to further and higher education and develop new practical skills in health and social care. Learning Outcomes After completing this SENCO : Special Educational Needs Coordination, the learner will be able to: Understand the roles and responsibilities of the Special Educational Needs Coordinator in the early years setting. Understand the strategies and techniques for supporting children and their families. Increase knowledge of SEN codes of practice. Why Choose Special Educational Needs Coordination Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials. Course developed by industry experts. MCQ quiz after each module to assess your learning. Automated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the course. ***Courses are included in this SENCO : Special Educational Needs Coordination Bundle Course*** Course 01: SENCO : Special Educational Needs Coordination Course 02: Level 2 Certificate in Understanding Children and Young People's Mental Health Course Course 03: Child Sexual Exploitation & Child Criminal Exploitation (CSE & CCE) Awareness Training Course 04: Level 2 Certificate in Understanding Common Childhood Illnesses Affecting Children Course 05: Care and support for Vulnerable Children Course 06: Level 2 Award in Babysitting Course 07: Advanced Diploma in Child Care ***Other Benefits of this Bundle Course*** Free 7 PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Free Retake Exam [ Note: Free PDF certificate as soon as completing the course ] Detailed course curriculum Module 1: Roles and Responsibilities of the Special Educational Needs Coordinator in the Early Years Understand the principles, statutory guidance and legislation underpinning practice for children with Special Educational Needs and Disability (SEND) in an early years setting Understand the role of the Early Years Special Educational Needs Coordinator (SENCo) Understand partnership working for the Early Years SENCo Understand early identification and action for children with SEND Module 2: Strategies and Techniques for Supporting Children and Families Understand the graduated approach in an early years setting Understand English as an additional language (EAL) Understand Education, Health and Care (EHC) plans ------------------------- ***Level 2 Certificate in Understanding Children and Young People's Mental Health Course Course Curriculum Module 1: Understand Children and Young People's Mental Health in Context Module 2: Understand Factors Which May Affect Children and Young People's Mental Health Module 3: Understand Children and Young People's Mental Health Concerns Module 4: Understand the Impact of Children and Young people's Mental Health Concerns Module 5: Understand How to Support Children and Young People with Mental Health Concerns ------------------------- ***Child Sexual Exploitation & Child Criminal Exploitation (CSE & CCE) Awareness Training Course Curriculum Module 01: An Overview of CSE and CCE Module 02: CSE: Risk Factors, Warning Signs, and Consequences Module 03: A Closer Look to CCE Module 04: Best Practice for Responding to Concerns ------------------------- ***Level 2 Certificate in Understanding Common Childhood Illnesses Affecting Children Course Curriculum Module 1: Understand How to Provide a Safe and Healthy Environment for Babies and Young Children Module 2: Understand Common Childhood Illnesses Module 3: Understand Health Emergencies and Specific Health Conditions in an Early Years Setting ------------------------- ***Care and support for Vulnerable Children Course Curriculum Module 01: Introduction Module 02: The Rights of Children Module 03: Understanding the Distinction Between Vulnerability and Risk Module 04: Child in Crisis Module 05: Controlling The Issues Module 06: Available Services Module 07: History and Future Developments ------------------------- ***Level 2 Award in Babysitting Course Curriculum Module 01: Accident Prevention and Fire Safety When Babysitting Module 02: Caring for Young Children in a Babysitting Environment Module 03: Craft Activities with Young Children Module 04: Respecting and Valuing Children ------------------------- ***Advanced Diploma in Child Care Course Curriculum Module 01: An Overview of Early Childhood Education Module 02: Empowering Children to Learn in the Early Years Module 03: The Process of Caring for Child's Development Module 04: Developing Socially and Emotionally Module 05: Aspects That Influence the Development Process Module 06: Stages of Language Development Module 07: The Process of Moral Development Module 08: Personality Development of Children Module 09: Problems During Infancy and Childhood Period Module 10: Adolescence's Problems Explained Module 11: Safeguarding Children ------------------------- Assessment Method After completing each module of the Special Educational Needs Coordination, you will find automated MCQ quizzes. To unlock the next module, you need to complete the quiz task and get at least 60% marks. Once you complete all the modules in this manner, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this SENCO course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This SENCO : Special Educational Needs Coordination is ideal for those already working in a SENCO role as part of their Early Years Practitioner role or interested in doing so. This course is also suitable for childminders. Requirements There are no specific requirements for this SENCO : Special Educational Needs Coordination because it does not require any advanced knowledge or skills. Students who intend to enrol in this SENCO : Special Educational Needs Coordination must meet the following requirements: Good command of the English language Must be vivacious and self-driven Basic computer knowledge A minimum of 16 years of age is required Career path SENCO : Special Educational Needs Coordination This SENCO : Special Educational Needs Coordination is appropriate for those who want to work in the following fields: Health and social care Childhood studies Community, youth and families Social work Early years Primary teaching Nursing Certificates Certificate of completion Digital certificate - Included

Residential Childcare: Residential Childcare Course Online Unlock the Power of Residential Childcare Course: Enrol Now! Our Residential Childcare Course is designed to help managers and leaders gain the knowledge and skills they need to work in residential care with children in a management role. This Residential Childcare course teaches you how to innovate and respond to a changing environment, as well as how to meet industry challenges and opportunities. Learn from expert tutors with industry experience, teaching you the latest expertise and best practice. This Residential Childcare Course is designed for Leadership and Management for Residential Childcare professionals who are aspiring to specialize in Leadership and Management for Residential Childcare. Enrol today and take the next step toward achieving your personal and professional goals. Courses you will get: Course 01: Level 5 Diploma in Leadership and Management for Residential Childcare Course 02: Level 4 Diploma in Child Psychology Course 03: Level 2 Safeguarding Children Training Course Course 04: Autism Diploma Course 05: Paediatric First Aid Course 06: Nursing & Prescribing [ Note: Free PDF certificate as soon as completing the Residential Childcare: Residential Childcare Course] Residential Childcare: Residential Childcare Course Online This Residential Childcare course consists of 20 modules. Assessment Method of Residential Childcare Course After completing Residential Childcare: Residential Childcare Course, you will get quizzes to assess your learning. You will do the later modules upon getting 60% marks on the quiz test. Apart from this, you do not need to sit for any other assessments. Certification of Residential Childcare Course After completing the Residential Childcare: Residential Childcare Course, you can instantly download your certificate for FREE. The hard copy of the certification will also be delivered to your doorstep via post, which will cost £13.99. Who is this course for? Residential Childcare: Residential Childcare Course Online This Residential Childcare: Residential Childcare Course is ideal for anyone in leadership or management within childcare. Including: - Apprenticeship in childcare management, Residential childcare assistant manager /Manager, assistant manager in within adult or children and young people’s social care settings. Requirements Residential Childcare: Residential Childcare Course Online To enrol in this Residential Childcare: Residential Childcare Course, students must fulfil the following requirements: Good Command over English language is mandatory to enrol in our Residential Childcare Course. Be energetic and self-motivated to complete our Residential Childcare Course. Basic computer Skill is required to complete our Residential Childcare Course. If you want to enrol in our Residential Childcare Course, you must be at least 15 years old. Career path Residential Childcare Course Online With the help of this Residential Childcare Course, you will be able to seek several promising career opportunities, such as: - Adult nurse. Care manager, Care worker, Health promotion/Education specialist, Health service manager, Learning disability nurse, Mental health nurse, Residential warden, Supervisor/Team leader, Volunteer manager, Care home manager.

Medicine: Medicine Course Online Unlock the Power of Medicine: Medicine Course: Enrol Now! Anyone working in care must understand how to correctly administer medication. This Medicine: Medicine Course is designed for anyone in a care role. This includes nurses, doctors, health care assistants, care home workers, childcare practitioners, and various other job roles where knowledge of medication is required. The Medicine: Medicine Course covers all of the knowledge needed for safe medication control, handling, and administration in social and domiciliary care settings. By the end of this Medicine: Medicine Course, the learner will understand prescribing, checking, handling, and administering medicines and the legislation behind this. Enrol on this Medicine: Medicine Course today to pursue your dreams and gain the experience, skills, and knowledge required to advance your professional development. Learning Outcomes After completing this Medicine: Medicine Course, the learner will be able to: Gain a thorough understanding of medication management. Understand the importance of medication management. Know the routes by which medicine can be administered. Understand the common issues in pharmacological management record-keeping requirements. Gain in-depth knowledge about palliative care. Gain a clear understanding of medicine optimisation and its four principles. Understand handling medication in residential aged care facilities. Understand the law and legislation concerning medicines. Why choose this Medicine: Medicine Course from the School of Health Care? Self-paced Medicine: Medicine course, access available from anywhere in the world. High-quality study materials that are easy to understand. Medicine: Medicine Course developed by industry experts. Free PDF certificate after completing the Medicine: Medicine Course. Courses you will get: Course 01: Control and Administration of Medicine Course 02: Nursing Assistant Diploma Course 03: Level 5 Diploma in Medical & Clinical Administration [ Note: Free PDF certificate will provide as soon as completing the Medicine: Medicine Course] Medicine: Medicine Course Online This Medicine: Medicine Course consists of 09 modules. Course Curriculum of Control and Administration of Medicine: Control and Administration of Medicine Module 01: Overview of Medicine Management Module 02: Significance of Medicine Management Module 03: The Best Way Medicine Can be Prescribed Module 04: Common Issues in Pharmacological Management Module 05: Administering Common Medicines Module 06: Defining Palliative Care Module 07: Medicine Optimisation and its Four Principles Module 08: Handling Medication in Residential Aged Care Facilities (RACFs) Module 09: Law and Legislation Concerning Medicines Assessment Method of Medicine: Medicine Course After completing Medicine: Medicine Course, you will get quizzes to assess your learning. You will do the later modules upon getting 60% marks on the quiz test. Apart from this, you do not need to sit for any other assessments. Certification of Medicine: Medicine Course After completing the Medicine: Medicine Course, you can instantly download your certificate for FREE. The hard copy of the certification will also be delivered to your doorstep via post, which will cost £13.99. Who is this course for? Medicine: Medicine Course Online This Medicine: Medicine Course is ideal for anyone who works in the Healthcare & Social Care setting. Including: Hospitals (both private and public) Clinical Commissioning Groups NHS Mental Health and Disability Services Nursing Homes & Hospices Residential Care Homes Requirements Medicine: Medicine Course Online To enrol in this Medicine: Medicine Course, students must fulfil the following requirements: Good Command over English language is mandatory to enrol in our Medicine: Medicine Course. Be energetic and self-motivated to complete our Medicine: Medicine Course. Basic computer Skill is required to complete our Medicine: Medicine Course. If you want to enrol in our Medicine: Medicine Course, you must be at least 15 years old. Career path Medicine: Medicine Course Online

Medical Teaching: 8 in 1 Premium Courses Bundle
By Compete High
The Medical Teaching: 8 in 1 Premium Courses Bundle offers a solid educational base for those seeking to better understand the healthcare field from a knowledge-sharing perspective. Covering key topics such as public health, biology, healthcare systems, and nursing, this bundle brings together essential subject matter for learners interested in both clinical understanding and medical instruction. With dedicated modules on paramedic training, phlebotomy, and laboratory technician roles, the bundle sharpens your awareness of healthcare operations. You’ll also explore pharmacy and lab procedures, all through an academic lens. It’s a great step for those considering medical education, or anyone needing a clearer grasp of healthcare functions without the need to locate a stethoscope. Learning Outcomes: Understand core healthcare topics and their academic frameworks Learn about nursing and paramedic theoretical responsibilities Explore biology relevant to healthcare and clinical settings Understand blood collection and phlebotomy techniques in theory Gain insights into pharmacy and laboratory technician duties Learn about public health systems and educational importance Who is this Course For: Future healthcare educators or medical tutors Nursing or public health theory enthusiasts Individuals aiming to teach clinical procedures Those pursuing roles in lab or technician sectors Biology learners focused on medical relevance Healthcare admin professionals exploring broader roles Paramedic theory learners with academic interests Pharmacy technician candidates studying online topics Career Path (UK Average Salaries): Medical Lecturer – £42,000/year Public Health Educator – £35,000/year Lab Technician – £25,000/year Phlebotomist – £23,000/year Pharmacy Technician – £26,000/year Paramedic (Theory Educator) – £37,000/year

Health Care Assistant HCA Training Mini Bundle
By Compete High
The Health Care Assistant HCA Training Mini Bundle is a well-rounded selection of courses tailored to those looking to step into care roles or understand core health topics. It features modules in healthcare, nursing, paediatric first aid, general first aid, and counselling — bringing together vital themes for both emotional and physical support in health settings. Whether you're exploring a future role or adding depth to existing knowledge, this online bundle balances care, communication, and compassion without over-promising. Clear content, structured delivery, and no unnecessary fluff — just what you need, when you need it. Learning Outcomes: Understand key responsibilities of a health care assistant. Learn core nursing concepts used in daily care settings. Explore how to offer emotional support through counselling basics. Study key principles of first aid and emergency response. Gain knowledge in paediatric health and first aid needs. Review safe care routines and communication in health roles. Who Is This Course For: Individuals considering health care support work. Entry-level care workers seeking structured online training. Those assisting vulnerable adults or children at home. Candidates preparing for NHS or private care roles. Parents and guardians wanting paediatric first aid training. Volunteers supporting community or residential services. Learners aiming to understand nursing and health basics. Anyone wanting to learn about care and emotional support. Career Path: Health Care Assistant – £21,000 to £28,000 Mental Health Support Worker – £22,000 to £30,000 Counselling Assistant – £20,000 to £27,000 Paediatric Nursing Assistant – £23,000 to £31,000 First Aid Responder – £21,000 to £29,000 Residential Care Worker – £20,000 to £28,000

ESAG Hands On Live Cases Training Course on Surgical Female Genitalia Cosmetic Procedures
4.8(18)By European Society of Aesthetic Gynecology ESAG
The European Society of Aesthetic Gynecology | ESAG is delighted to present Hands On, Live Cases Training Courses on Surgical Female Genitalia Cosmetic Procedures with a fully rejuvenated scientific program Alexandros Bader, MD, FAAOCG, FAACS Ob&Gyn Consultant (London - Dubai) Specialist Pelvic Floor Reconstruction – Cosmetic Gynecology President and Founder of the European Society of Aesthetic Gynecology –ESAG Founder and Co-director of the Bader Medical Institute of London Associate researcher at University of Oxford-UK SURGICAL Hands-On Training: December 11th to 13th: This is a unique opportunity to work side-by-side with Dr Bader, pioneer in the field of aesthetic gynecology and one of the finest Surgeons in the world in the field Of Reconstructive and Aesthetic Gynecology. A surgeon who counts more than 3500 surgical procedures in his portfolio for the last 15 years. Theory of Surgical Cosmetic & Aesthetic Gynecology Hands on, Live cases training on Surgical Female Genital Cosmetic & Reconstructive Gynecology procedures Covered Surgical Topics: · Labia Minora Plasty · Labia Majora Plasty · Clitoral Hoodectomy · Vaginal Tightening with Single Thread technique · Fat grafting to genital area

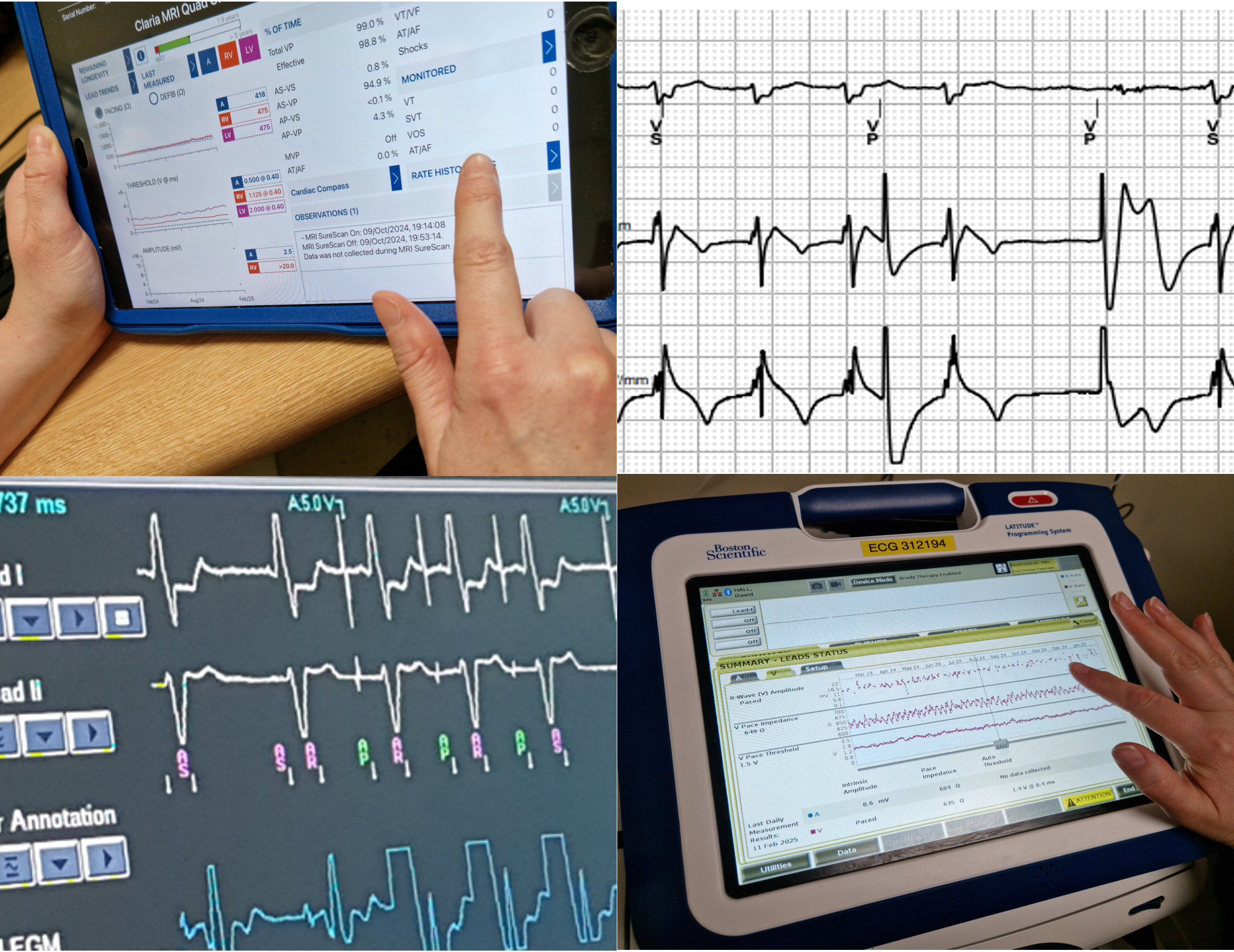
Demystifying cardiac devices — a practical introduction for healthcare professionals new to device therapy and monitoring A one day introductory course for all healthcare professionals with limited or no experience of cardiac devices. Ever wanted to know why there are so many different devices and what they do? What do device checks even involve? When are they needed? What should I be looking for on my telemetry? The course will be hosted by senior Cardiac Physiologists/ Scientists from the Cardiac Rhythm Management team at Manchester University NHS Foundation Trust. Full draft agenda below. **If submitting payment via CPD funding OR Purchase Order (i.e. not a card payment) - please DO complete your registration - you can detail payment method in the ‘Billing name' box. Many thanks. **
Level 6 Diploma Pharmacy Assistant Dispenser and Pharmacy Technician - CPD Certified
By Wise Campus
Pharmacy Technician Training and Pharmacy Assistant Dispenser To ensure that patients get the greatest benefit from their medications, pharmacy technicians are crucial to the efficient operation of pharmacies. Do you care deeply about protecting life as a Pharmacy Technician ? Do you want to voluntarily assist others? Don't wait any longer, then. Enrol in our specialised training for pharmacy technicians to improve your skills and advance your career in this area. Because of the vital function that pharmacy technicians play in the healthcare system, there is still a high need for pharmacy technicians in the UK. The demand for qualified Pharmacy Technicians is anticipated to increase further due to the expanding population and growing reliance on healthcare services. This sector is excellent for launching a career, particularly for someone wishing to start out as a Pharmacy Technician. Yet, you'll need sufficient training and the right direction to become an effective pharmacy technician. You will be prepared to work as a knowledgeable pharmacy technician after completing this training. You can gain a thorough understanding of a pharmacy technician's position and responsibilities by taking our Pharmacy Technician course. By taking our pharmacy technician course, you will be able to develop strong communication skills that will enable you to interact comfortably with patients and healthcare professionals. The Pharmacy Technician course will teach you how to clearly understand inventory management, standard operating procedures, and dispensing techniques. The Pharmacy Technician course also covers risk management, pharmacy prescription, dispensing, and minimising dispensing errors. You should enrol in our Pharmacy Technician course as soon as possible if you wish to practise at the highest level of pharmacy. Main Course: Pharmacy Technician Training Free Courses are including with this Pharmacy Technician: Pharmacy Technician Training Course Along with The Pharmacy Technician Training Course, We Offer a free Pharmacy Assistant course Along with The Pharmacy Technician Training Course, We Offer a free Medical Terminology Course Along with The Pharmacy Technician Training Course, We Offer a free Medical Transcription Course Along with The Pharmacy Technician Training Course, We Offer a free Control and Administration of Medicine Course Along with The Pharmacy Technician Training Course, We Offer a free Level 5 Diploma in Medical & Clinical Administration Course Along with The Pharmacy Technician Training Course, We Offer a free Nursing Assistant Diploma Course Along with The Pharmacy Technician Training Course, We Offer a free Level 2 Award in the Prevention and Control of Infection Course Special Offers of this Pharmacy Technician: Pharmacy Technician Training Science Course This Pharmacy Technician: Pharmacy Technician Training Course includes a FREE PDF Certificate. Lifetime access to this Pharmacy Technician: Pharmacy Technician Training Course Instant access to this Pharmacy Technician: Pharmacy Technician Training Course Get FREE Tutor Support to this Pharmacy Technician: Pharmacy Technician Training Course Pharmacy Technician Training and Pharmacy Assistant Dispenser By making sure that your community knows they can count on you for assistance and top-notch customer service, you can make a significant contribution to the UK healthcare system. You will learn everything you need to know in this course on pharmacy technician: pharmacy technician to support and stabilize your team as a pharmacy technician or pharmacy technician assistant. Who is this course for? Pharmacy Technician Training and Pharmacy Assistant Dispenser In order to gain the expertise, aspiring professionals might benefit from the Pharmacy Technician Training course. Anyone can become more familiar with the skill sets needed to seek a job as a pharmacy technician by taking a pharmacy technician course. Requirements Pharmacy Technician Training and Pharmacy Assistant Dispenser To enrol in this Pharmacy Technician: Pharmacy Technician Training Course, students Need: To join in our Pharmacy Technician: Pharmacy Technician Training Course, you must have a strong command of the English language. To successfully complete our Pharmacy Technician: Pharmacy Technician Training Course, you must be vivacious and self driven. To complete our Pharmacy Technician: Pharmacy Technician Training Course, you must have a basic understanding of computers. A minimum age of 15 is required to enrol in this Pharmacy Technician: Pharmacy Technician Training Course. Career path Pharmacy Technician Training and Pharmacy Assistant Dispenser A pharmacy technician course's objective is to give you the skills and knowledge you'll need to be successful in the healthcare industry. Hospitals, community pharmacies, nursing homes, and the pharmaceutical sector all offer opportunities for employment as pharmacy technicians.

Search By Location
- Nursing Courses in London
- Nursing Courses in Birmingham
- Nursing Courses in Glasgow
- Nursing Courses in Liverpool
- Nursing Courses in Bristol
- Nursing Courses in Manchester
- Nursing Courses in Sheffield
- Nursing Courses in Leeds
- Nursing Courses in Edinburgh
- Nursing Courses in Leicester
- Nursing Courses in Coventry
- Nursing Courses in Bradford
- Nursing Courses in Cardiff
- Nursing Courses in Belfast
- Nursing Courses in Nottingham
