- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
8164 Medicine & Nursing courses
Description Food Technology Diploma In today's digital era, the global food industry is undergoing dramatic shifts. From the biotechnological innovations enhancing food quality to the ethical imperatives of sustainable production, the industry's landscape is evolving at an unprecedented rate. If you're eager to be at the forefront of these advancements, the online Food Technology Diploma is tailor-made for you. This comprehensive online course takes you on a journey through the pivotal aspects of food technology. Beginning with an insightful introduction to Food Technology, you'll gain a foundational grasp of the key concepts and applications that define this field. As the course progresses, you'll explore essential topics like Food Safety and Preservation Techniques, ensuring that you have the knowledge and skills to manage food quality, longevity, and safety. One of the key components of the Food Technology Diploma is the segment on Food Processing and Machinery. Here, you'll become well-acquainted with the machines and processes that are at the heart of the food industry. Whether it's mechanised sorting or high-tech processing, you'll gain a clear understanding of how these innovations contribute to the modern food supply chain. Of course, the sensory aspect of food cannot be overlooked. In the module on Sensory Evaluation and Food Quality, you'll be equipped to assess and judge food items based on their sensory attributes. This ensures that, whether you're involved in food production or research, you're well-versed in delivering products that resonate with consumers' preferences. The Biotechnology Revolution in Food is another intriguing area covered in this diploma. Biotechnology is undoubtedly transforming how we think about, produce, and consume our food. You'll uncover the latest advancements and understand their implications for both the industry and consumers. For those with a keen interest in the health implications of what we eat, the Nutrition and Functional Foods section offers a deep understanding of how food impacts our well-being. This is coupled with insights into Sustainable and Ethical Food Production, emphasising the importance of producing food that not only feeds the world but does so in a responsible manner. Yet, this Food Technology Diploma doesn't stop at the tangible. Recognising the influence of the digital age on the food industry, the course delves into The Digital Transformation of the Food Industry. This segment sheds light on how digital tools and platforms are reshaping everything from food distribution to consumer engagement. In the Global Trends and Innovations in Food module, learners are introduced to the ever-changing dynamics of the worldwide food market. This ensures you remain abreast of the latest developments, ready to innovate and adapt. Finally, what does the future hold for food technology? The Future of Food Technology section provides a forward-thinking perspective on what lies ahead, arming you with the foresight to navigate the challenges and opportunities on the horizon. In essence, the Food Technology Diploma is a holistic, online course designed for those passionate about the food industry's present and future. With its blend of practical knowledge and futuristic insights, this diploma prepares you for a rewarding journey in the world of food technology. Enrol now and embark on an educational experience that promises both depth and breadth in this dynamic field. What you will learn 1: Introduction to Food Technology 2: Food Safety and Preservation Techniques 3: Food Processing and Machinery 4: Sensory Evaluation and Food Quality 5: The Biotechnology Revolution in Food 6: Nutrition and Functional Foods 7: Sustainable and Ethical Food Production 8: The Digital Transformation of the Food Industry 9: Global Trends and Innovations in Food 10: The Future of Food Technology Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

Description Neuroscience Diploma In biology, a sub-field known as neuroscience is concerned with analysing the structure of our nervous system, how it functions, and how it has evolved. Neuroscience is a constantly changing discipline; however, its contents may be categorised into various branches, such as clinical, cognitive, developmental, and computational neuroscience. It is an interdisciplinary study closely associated with physics, chemistry, medicine, computer science, linguistics, philosophy, and engineering. Neuroscientists concentrate on analysing the nervous system at both the cellular and operational levels to see how it impacts behavioural events and patterns. Their research may include hypothesising theories or conducting laboratory experiments using special techniques, tools, or computer simulations. In the Neuroscience Diploma course, students will learn how to examine the nervous system, including its structure and biological processes, and how these apply to the development of artificial intelligence systems. There are different specialisations that people can choose within the field, such as behavioural and cognitive, affective, molecular, neuroimaging, neurolinguistics, neurophysiology, social neuroscience, and many more. Most jobs available for someone with a background in neuroscience would mostly be in the healthcare and pharmaceutical industries, with companies that handle communication and information science, and with organisations that manage public health structures. Traditionally, the discipline of neuroscience had birthed professionals like neuroanatomists, neurologists, neuro-pharmacologists, neurological surgeons, technicians dealing with electro-neurodiagnostic machines, and psychiatrists. Students will learn about the nervous system's neurophysiology and functional organisation to better understand human behaviour. Find out how neural systems work in our brain and spinal cord to help us detect things, move different parts of our bodies, store information we obtain from our senses, relate it to emotions, and analyse it using various cognitive processes. The main objective of this Neuroscience Diploma course is to help students establish the foundational knowledge that will help them understand how sensations may be impaired, how injuries may lead to physical or cognitive difficulties and other diseases or malfunctioning of the nervous system. Through the Neuroscience Diploma course, you will come to know how neurons transmit information among themselves using electrical impulses. This course is an introductory course on the functioning and organisation of the central nervous system. Various topics will be covered, including the structure of neurons, the neurotransmitters in the brain, electrical impulses as signals, motor and sensory systems, the neurobiological aspect of human development, simple circuits, learning, and human behaviour. What you will learn 1:A Walkthrough of Your Nervous System 2:Know About Your Brain and Spinal Cord 3:Sensory Neuron and The Skin 4:Role of Eye in Vision 5:The Auditory System 6:Taste and Smell 7:Movement 8:Know About Intelligence Emotions and Consciousness 9:The Role of Brain in Language 10:Role of Brain in Learning and Memory Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Description Immunology Diploma A comprehensive online course designed for those who wish to deepen their understanding of the immune system and its myriad functions. This online course is an excellent opportunity for both professionals in the medical field and enthusiasts who are keen to grasp the intricate workings of the body's defence mechanisms. The course kicks off with 'The Immune System: An Overview', providing learners with a foundational grasp of the system's components and functions. From there, the focus shifts to 'Innate Immunity: The First Line of Defence', which delves into the body's immediate and non-specific defences against pathogens. Moving forward, the Immunology Diploma explores 'Adaptive Immunity: A Tailored Response'. This module allows learners to comprehend how our body crafts a specific and potent response against foreign invaders and keeps a memory of past intruders for quicker action in future encounters. The journey continues with 'Immune Response Regulation', an essential topic that underscores how the body balances its immune reactions to prevent undue damage to self-cells and tissues. Any malfunction in this regulation leads to conditions such as autoimmune diseases, which are thoroughly covered in the section 'Autoimmunity and Autoimmune Diseases'. It offers an in-depth look into how, at times, the immune system might mistakenly target its own cells, leading to various health complications. A highlight of the Immunology Diploma is the segment on 'Immunodeficiency Disorders'. Here, learners will uncover the situations where the immune system's functioning falls short, leaving the body vulnerable to infections and diseases. 'Allergies and Hypersensitivity' is another crucial module that provides insights into the body's exaggerated responses to certain harmless substances, causing symptoms ranging from mild itching to severe anaphylaxis. The subject of transplantation has always been a matter of great intrigue in the medical community. 'Immunology of Transplantation' will equip learners with knowledge about how the immune system reacts to transplanted organs and tissues and the measures taken to ensure successful transplantation. In today's world, infectious diseases remain a significant concern. The module 'Immunology of Infectious Diseases' navigates through the complex relationship between pathogens and the immune system. It sheds light on how the body fights off various infections and the challenges it faces. Concluding the course is 'Immunotherapy and Vaccines', a timely and paramount topic. Learners will gain an understanding of the principles behind harnessing the immune system to combat diseases and the development and working of vaccines that protect us from several life-threatening conditions. In summary, the Immunology Diploma offers a well-structured and comprehensive online learning experience, tailor-made for individuals who seek to gain a profound understanding of the immune system. This course promises to be an enriching journey through the world of immunology, equipping learners with knowledge that is both theoretical and applicable in real-world scenarios. Enrol now and embark on a fascinating journey through the human body's unparalleled defence system. What you will learn 1:The Immune System: An Overview 2:Innate Immunity: The First Line of Defence 3:Adaptive Immunity: A Tailored Response 4:Immune Response Regulation 5:Immunodeficiency Disorders 6:Autoimmunity and Autoimmune Diseases 7:Allergies and Hypersensitivity 8:Immunology of Transplantation 9:Immunology of Infectious Diseases 10:Immunotherapy and Vaccines Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Description Neuroplasticity Diploma Neuroplasticity Diploma offers a comprehensive online journey into the fascinating world of the brain's ability to change and adapt. This innovative course is designed for those who wish to deepen their understanding of how the brain works, evolves, and reshapes itself throughout a person's lifetime. Beginning with an Introduction to Neuroplasticity, this diploma breaks down the complexities of the brain's dynamic nature. Rather than seeing the brain as a static organ, participants will come to appreciate the nuances of its growth, adaptation, and its boundless potential for transformation. In the Sensory Substitution section, learners will explore how the brain can repurpose one sense to compensate for the loss or inefficiency of another. Imagine a world where the blind can 'see' using their sense of touch or where the deaf might 'hear' via visual cues. The marvels of sensory substitution make such wonders possible, and this section unpacks the science behind it. Developmental Plasticity addresses the evolution of the brain during different stages of human growth. From infancy to adulthood, the brain undergoes significant changes, constantly adapting and learning. This section provides invaluable insights into how early experiences can shape our cognitive abilities and behaviours for years to come. In Synaptic Plasticity, the course delves into the cellular level of brain adaptation. Unravel how individual neural connections strengthen or weaken in response to experiences, laying the foundation for memory, learning, and behavioural change. The Seven Rules of Neuroplasticity is an illuminating segment, guiding learners through the fundamental principles governing how the brain changes. With these rules, one can better understand the underlying mechanisms and perhaps even harness them to foster positive personal growth. Brain Training, a popular topic in today's digital age, seeks to explore the tools and techniques that claim to enhance cognitive functions. With so many apps and programmes promising to 'train' the brain, this section offers a scientific lens to evaluate their efficacy and potential. One cannot discuss neuroplasticity without addressing Nerve Injury and Brain Damage. This section offers insights into how the brain recovers and compensates after trauma, showcasing its resilience and adaptability. Addiction and Pain, two formidable challenges in neuroscience, are scrutinised in the context of neuroplasticity. This section seeks to offer an understanding of how repeated behaviours can reshape the neural pathways, leading to addiction, and how pain, both physical and emotional, can change the brain's structure and function. Lifelong Changes in the Brain provides hope and inspiration by highlighting that the brain's ability to adapt isn't confined to the early years but is a lifelong phenomenon. Be it learning a new skill in one's golden years or adapting to unforeseen life changes; the brain's plasticity supports us throughout our journey. The course rounds up with a Conclusion, where learners are equipped with a holistic understanding of neuroplasticity. The Neuroplasticity Diploma doesn't just offer knowledge; it provides a foundation for learners to apply this knowledge in their personal and professional lives, be it in therapy, education, or personal development. In essence, the Neuroplasticity Diploma is an online treasure trove for anyone keen on grasping the immense capabilities of the human brain. Enrol today and embark on a journey of discovery, wonder, and infinite possibilities. What you will learn 1:Introduction to Neuroplasticity 2:Sensory Substitution 3:Developmental Plasticity 4:Synaptic Plasticity 5:The Seven Rules of Neuroplasticity 6:Brain Training 7:Nerve Injury and Brain Damage 8:Addiction and Pain 9:Lifelong Changes in the Brain 10:Conclusion Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Description Fundamentals of Quantum Physics Diploma Introducing the Fundamentals of Quantum Physics Diploma, a comprehensive online course tailored for individuals who aspire to grasp the core concepts of quantum physics. Whether you're a student, an enthusiast, or a professional seeking to enhance your knowledge, this course is designed to provide you with a deep understanding of the pivotal principles and historical developments in the realm of quantum physics. The Fundamentals of Quantum Physics Diploma starts with an enlightening 'Introduction to Quantum Physics'. As learners progress, they'll navigate through the fascinating world of 'Particles, Waves, and Lights', providing them with a robust foundation to comprehend the nuances of the quantum realm. Every scientific revelation has a story. The course takes learners on a journey through 'The Birth of the First Quantum'. It allows them to explore 'The First Quantum Concept', laying down the groundwork for the myriad of quantum phenomena they'll encounter. No study of quantum physics is complete without acknowledging the pivotal role of Max Planck. The course dedicates an entire module to 'Max Planck, the Father of Quantum Theory', ensuring learners appreciate the monumental contributions of this iconic figure. His work serves as the cornerstone for many quantum principles taught throughout the diploma. With the 'Uncertainty and Superposition' module, learners will gain insights into the unpredictability and the fascinating principle of superposition, where particles can exist in multiple states simultaneously. This principle has confounded and fascinated physicists for decades, and through this course, learners will get a chance to unravel its mysteries. The course seamlessly integrates Einstein's theories with a focus on 'Einstein's Relativity'. Understanding relativity is crucial for those wanting to master quantum physics, and the Fundamentals of Quantum Physics Diploma ensures this topic is explored in-depth. While quantum physics has its unique laws and principles, it also touches on more universal themes like 'The Law of Attraction'. Learners will discover the quantum underpinnings of this law and how it has been interpreted and debated in the scientific community. 'Causality in Quantum Physics' is another compelling module that sheds light on the cause-effect relationship in the quantum world. It presents a fresh perspective and challenges conventional thinking, pushing learners to view quantum physics from a novel angle. Lastly, but certainly not least, the course delves into the intriguing topic of 'Quantum Mechanics and General Relativity Incompatibility'. This module provides learners with insights into the ongoing challenges and debates surrounding the reconciliation of quantum mechanics with Einstein's theory of general relativity. In conclusion, the Fundamentals of Quantum Physics Diploma offers a thorough and engaging online learning experience. It's structured to ensure that learners not only acquire theoretical knowledge but also develop an appreciation for the historical context and the pioneers behind quantum revelations. Embark on this enlightening journey and gain a formidable understanding of the quantum universe. Your quantum adventure awaits. What you will learn 1:Introduction to Quantum Physics 2:Particles, Waves, and Lights 3:The Birth of the First Quantum 4:The First Quantum Concept 5:Max Planck, the Father of Quantum Theory 6:Uncertainty and Superposition 7:Einstein's Relativity 8:The Law of Attraction 9:Causality in Quantum Physics 10:Quantum Mechanics and General Relativity Incompatibility Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Description Microbiology Diploma Microbiology is a branch of biology that deals with the study of microorganisms. Microorganisms are extremely small forms of life like bacteria, protozoa, parasites, viruses and fungi. These organisms are microscopic i.e. they cannot be seen with the naked eye. There is often a misconception that microorganisms are dangerous and cause diseases. In reality, microorganisms are mostly beneficial or have a neutral effect in our lives. Those microorganisms or microbes that cause diseases are known as pathogens. Microorganisms are present everywhere but we are not able to see it because of their tiny sizes. Microbiologists investigate this hidden world of organisms that influence our environment, nutrition and health. Interestingly enough, there are more microbial cells in our bodies than our own cells. Microbiology includes several disciplines such as virology (study of viruses), bacteriology (study of bacteria), mycology (study of fungi), and parasitology (study of parasites). It is using tools like microscopes, genetics and culturing that microbiologists examine microorganisms. Genetics and molecular biology throws light into evolutionary relationships between microorganisms and their habitats. Culturing is the process of cultivating microorganisms to understand them better. Microbiology Diploma is a course is an introductory course especially for beginners to understand microbiology and its tricky concepts better. Microbiology Diploma presents insights on forms, functions and impacts of microorganisms in nature and on human lives. The characteristics, distinguishing features, processes and diversity are taken up in Microbiology Diploma. What you will learn 1: Introduction to Microbiology 2: Microbiology as a Young Science 3: Introduction to Microbes 4: Cell Structure and Functions 5: Introduction to Metabolism 6: Microbial Genetics 7: Microbial Growth 8: Microbial Ancestry 9: Harnessing Energy, Fixing Carbon 10: Respiration and Fermentation Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

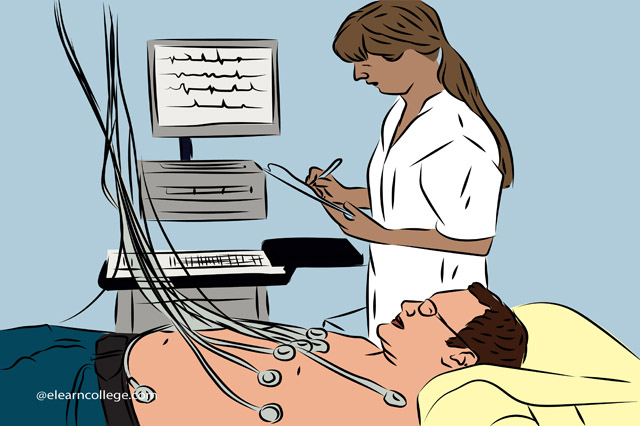
Description Ecg Assessment And Interpretation Diploma Unleash the world of cardiac rhythms and enhance your skillset with the Ecg Assessment And Interpretation Diploma. This online course offers a comprehensive overview of electrocardiography, extending beyond mere theory to ensure practical expertise. Offering complete flexibility, this course lets learners progress at their own pace, and is perfect for healthcare professionals looking to enrich their knowledge, or students aspiring to a career in healthcare. At the heart of the Ecg Assessment And Interpretation Diploma lies an in-depth exploration of Physiology and Cardiac Anatomy. The course delves into the structure and function of the heart, detailing the fundamentals that all healthcare practitioners should grasp. This essential foundation supports a solid understanding of the electrical workings within the heart, and how these translate into the waveforms we see on an ECG. Building on this foundation, the course proceeds to 'Getting a Rhythm Strip', exploring the techniques for obtaining accurate ECG readings. It covers the practical aspects of patient preparation and placement of ECG leads, critical factors in ensuring clear, interpretable readings. The course then seamlessly transitions into 'Interpreting Rhythm Strips', providing an analytical approach to recognise and understand common rhythm abnormalities. The Ecg Assessment And Interpretation Diploma then delves into the world of arrhythmias, offering comprehensive modules on Sinus Node, Atrial, Junctional, and Ventricular Arrhythmias. Here, learners will become well-versed in the identification and significance of various types of cardiac arrhythmias. The complex topic of Atrioventricular Blocks also gets thorough coverage, enlightening students on the nature and clinical implications of these conditions. As an invaluable addition to the curriculum, 'Treating Arrhythmias' imparts knowledge of interventions, from medication to pacing and defibrillation. This essential knowledge completes the full circle of ECG interpretation, leading to appropriate and effective treatment strategies. The pinnacle of the Ecg Assessment And Interpretation Diploma is the module on 'Obtaining and Interpreting 12-lead ECG'. This vital section goes beyond basic rhythm strips to a full 12-lead ECG, expanding the learner's proficiency in assessing and interpreting complex cardiac conditions. Overall, the Ecg Assessment And Interpretation Diploma is a thorough and detailed course, designed for individuals who seek a robust understanding of electrocardiography. The online learning platform allows learners to interact, ask questions, and clarify doubts, fostering an engaging, productive learning environment. Take the first step towards mastering ECG assessment and interpretation, and sign up for this diploma course today. With the Ecg Assessment And Interpretation Diploma, the complex world of cardiac rhythms becomes an open book, bringing invaluable skills within your reach. What you will learn 1:Physiology and Cardiac Anatomy 2:Getting a Rhythm Strip 3:Interpreting Rhythm Strips 4:Sinus Node Arrhythmias 5:Atrial Arrhythmias 6:Junctional Arrhythmias 7:Ventricular Arrhythmias 8:Atrioventricular Blocks 9:Treating Arrhythmias 10:Obtaining and Interpreting 12-lead ECG Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Description Endometriosis Awareness Diploma The medical condition known as Endometriosis affects about 2%-10% of women of a particular age range- those who can conceive. It is a medical gynaecological condition that is quite common. The etymological root of the term is 'endometrium', a name for the lining tissue of the uterus. The tissue known as the endometrium builds up and gets thicker during a menstrual cycle. These tissues shed and come out as part of period blood if the woman does not get pregnant. When women have Endometriosis, tissues that look like endometrium develop not inside but outside the uterus. These can be on other organs of the reproductive system in the pelvic area or the woman's abdominal cavity. As the menstrual cycle goes on and hormonal changes occur, these tissues respond to the hormones every month. As endometrial tissues build up, they do too. This results in bleeding in the pelvis, although in small amounts. This can cause inflammation and swelling, and subsequent scarring in the normal tissues around them. If the ovary is affected, a fibrous cyst known as a blood blister may be formed, a condition known as endometrioma. The Endometriosis Awareness Diploma course has been developed to equip learners with a clear understanding of this medical condition that affects a significant percentage of women. The Endometriosis Awareness Diploma course includes the causes, symptoms, and how these symptoms show up. The course also consists of the diagnosis and treatment of the condition. The Endometriosis Awareness Diploma course starts by discussing what exactly Endometriosis is. This also provides general information about menstrual cycles. There are different kinds of Endometriosis, which will be looked at along with their causes, the signs and symptoms, their different stages, and the various risk factors that come with the medical condition. Other topics, like the diagnosis process, including the available methods and benefits, are also covered in the Endometriosis Awareness Diploma course. The Endometriosis Awareness Diploma course deepens into laparoscopy- the most common practice in diagnosing Endometriosis. Other treatments such as hormonal treatment and surgical processes for treating inflammation or removing tumours caused by Endometriosis will also be discussed. The Endometriosis Awareness Diploma course also provides basic knowledge regarding analgesic treatment and treatments that use artificial hormones for follicle stimulation, surgical procedures that help treat infertility, IVF (In-Vitro Fertilization), intrauterine insemination, and Assisted Reproduction Technology. These processes will be learnt in-depth along with their statistics regarding success. Changes in diet that can be made to alleviate endometriosis symptoms and yoga routines that can relieve the symptoms will also be looked at. The course includes case studies to learn from real-life cases and better know symptoms and treatments. This course can teach you many things about this medical condition, including some of which are listed below: 1 Develop an understanding of Endometriosis, the different kinds, their causes and symptoms, various stage of the condition, and the risk factors involved. 2 Learn about the diagnosis and treatment of the condition. Learn about the available treatments that will help in fertilization. 3 Learn about dietary changes which will reduce or alleviate the symptoms of the condition. What you will learn 1: A brief introduction to endometriosis 2: The menstrual cycle and endometriosis 3: Endometriosis and other parts of the body 4: Endometriosis and infertility 5: Diagnosing endometriosis 6: Drugs to treat endometriosis 7: Surgery and endometriosis 8: Endometriosis and alternative treatments 9: Chronic pain due to endometriosis 10: Coping and living with endometriosis Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

Description Dental Materials Science Diploma Introducing the Dental Materials Science Diploma, an online course tailored for those eager to master the intricacies of dental materials. As the world of dentistry evolves, it's essential for professionals to stay abreast of the latest advancements and materials used in the field. This course provides a comprehensive understanding of the various materials utilised in modern dentistry and the science behind them. The Dental Materials Science Diploma kicks off with an in-depth introduction to Dental Materials Science. This foundation ensures that learners gain a robust understanding of the basic concepts, paving the way for more advanced topics. One of the primary focuses is on Metals in Dentistry. While metals have been used in dental practices for decades, it's crucial to understand their properties, advantages, and potential drawbacks. This module examines the myriad of metals used, their applications, and the ongoing research in this arena. Polymers and Resins in Dentistry is another pivotal topic covered in this course. As dentistry progresses, polymers and resins have found increased applications due to their versatile properties. Learners will explore the types, applications, and considerations when using these materials. Another integral component of modern dentistry is the use of Ceramics in Dental Applications. This module delves into the types of ceramics used, their strengths, and how they've revolutionised certain dental procedures. Their aesthetic and functional advantages make them a popular choice in restorative dentistry. The Dental Materials Science Diploma also sheds light on Dental Cements and Adhesives. These often-overlooked materials play a vital role in procedures ranging from fillings to crowns. Their bonding properties and the science behind them are explored in depth. As the dental industry progresses, the role of Biomaterials in Implant Dentistry becomes more pronounced. This module explores the myriad of biomaterials used in implants, their advantages, potential complications, and the cutting-edge research shaping their future. The course then transitions to Material Selection and Clinical Considerations. It's not just about knowing the materials but also about understanding when and where to use them. This segment ensures that learners are equipped to make informed decisions in a clinical setting. Given the nature of the materials and their close interaction with the human body, Infection Control in Dental Materials is an essential module. It underscores the significance of hygiene, sterilisation techniques, and best practices to minimise infection risks. In a rapidly evolving field, staying updated is crucial. The Technological Advances in Dental Materials segment introduces learners to the latest advancements, tools, techniques, and materials revolutionising dentistry. Lastly, the course highlights the importance of sustainability in the dental sector with Dental Materials: Sustainability and Environmental Impact. In an age where sustainability is paramount, it's vital for dental professionals to understand the environmental footprint of the materials they use and ways to minimise their impact. In summary, the Dental Materials Science Diploma is a comprehensive online course designed for individuals keen to expand their knowledge in the realm of dental materials. With a blend of foundational knowledge and advanced insights, this course is poised to elevate one's expertise in the world of dentistry. What you will learn 1:Introduction to Dental Materials Science 2:Metals in Dentistry 3:Polymers and Resins in Dentistry 4:Ceramics in Dental Applications 5:Dental Cements and Adhesives 6:Biomaterials in Implant Dentistry 7:Material Selection and Clinical Considerations 8:Infection Control in Dental Materials 9:Technological Advances in Dental Materials 10:Dental Materials: Sustainability and Environmental Impact Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.

Description Physics Foundation Diploma We live in the century of science and technology, and the introduction of science has transformed our lives. Even when we had no idea about science, our lives were governed by the principles of science and its various branches. Physics governs our everyday lives and is involved in everything we do. Physics is often considered an immersive yet challenging subject. It is among the most fundamental scientific disciplines. This branch of science deals with the nature of matter and energy and its various applications. In our Physics Foundation Diploma course, you will explore the fundamental aspects of physics in a very interactive format. This program will help you understand the nature and scope of the subject. In addition to having a basic understanding of the subject, you will be introduced to topics such as Motion, Vectors, Speed and Acceleration, Circular motion, Force, Friction and Gravity, Work, Impulse and Momentum, Simple Harmonic Motion, and the concept of relativity. This Physics Foundation Diploma course is very beneficial for students studying in high schools, colleges and universities, having physics as a subject. This program will ensure that you know the basics before you go deep into the subject. Physics shares a fundamental understanding of how the world around us works, everything from the natural to the technological devices we depend on daily. It helps us contemplate profoundly and think critically to satisfy our curious minds. We, as humans, have the natural tendency to look through things. We never see things for what it is. We saw volcanos, rains, waves, or cyclones, we did not take them for what we just saw, but we looked into them. This curiosity is what makes us who we are. Being one of the fundamental branches of science, physics opens a door in front of us, a door to see the world for what it is. This physics foundation diploma course will open your eyes and, in turn, opportunities for you, irrespective of your field and academic background. What you will learn 1: Understanding Physics 2: Vectors 3: Speed and Acceleration 4: Circular Motion 5: Force 6: Friction and Gravity 7: Work 8: Impulse and Momentum 9: Simple Harmonic Motion 10: Wonders of Relativity Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
Search By Location
- Medicine & Nursing Courses in London
- Medicine & Nursing Courses in Birmingham
- Medicine & Nursing Courses in Glasgow
- Medicine & Nursing Courses in Liverpool
- Medicine & Nursing Courses in Bristol
- Medicine & Nursing Courses in Manchester
- Medicine & Nursing Courses in Sheffield
- Medicine & Nursing Courses in Leeds
- Medicine & Nursing Courses in Edinburgh
- Medicine & Nursing Courses in Leicester
- Medicine & Nursing Courses in Coventry
- Medicine & Nursing Courses in Bradford
- Medicine & Nursing Courses in Cardiff
- Medicine & Nursing Courses in Belfast
- Medicine & Nursing Courses in Nottingham