- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7576 Medicine & Nursing courses delivered Online
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
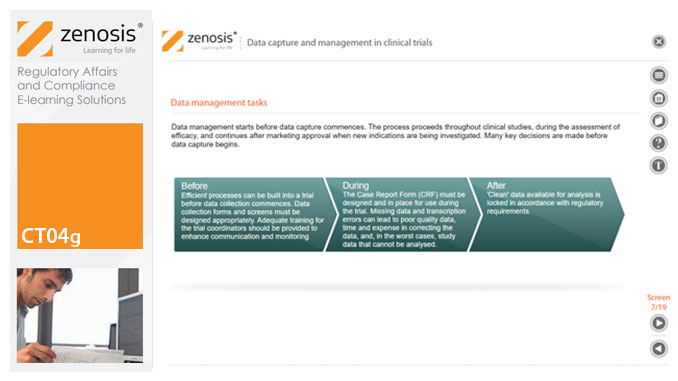
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
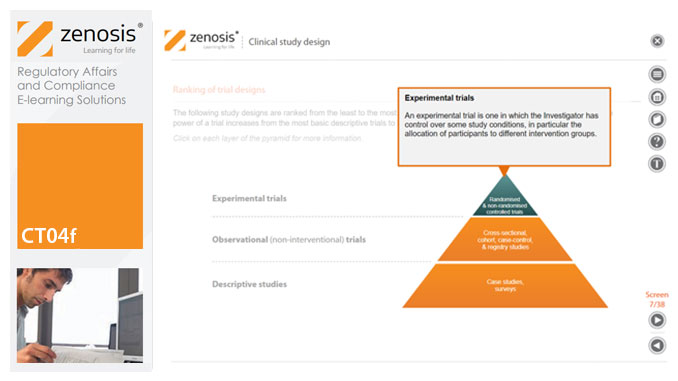
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT03e - Clinical trial investigator’s GCP responsibilities
By Zenosis
A clinical investigator is responsible for conducting the clinical trial in compliance with the study protocol, GCP, medical ethics, and applicable legal requirements. The clinical research community expects that investigators and clinical staff are fully trained in GCP. Duties and functions discussed in this short course include: provision of adequate resources; liaison with IRB/IEC; compliance with protocol; management of investigational product(s), informed consent and data records; and safety reporting.
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
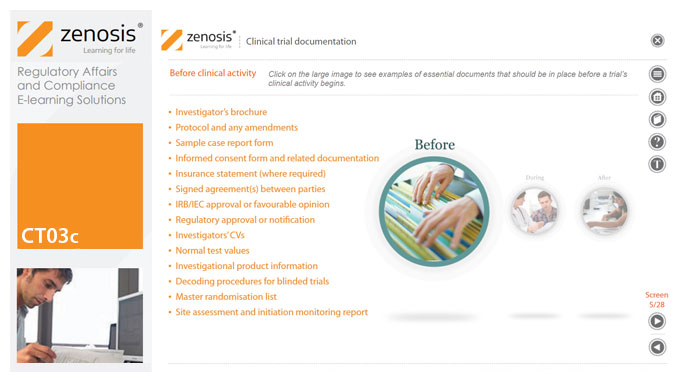
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
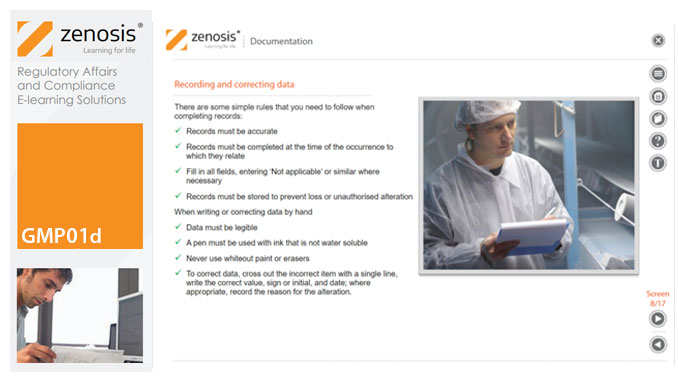
GMP01d - Documentation
By Zenosis
Comprehensive documentation of procedures, formulas, work instructions, and specifications, and thorough recording of batch data, are fundamental requirements of GMP. In this short course we explain why documentation is so important, identify different types of document required, and set out some simple rules for recording and correcting data.
CT03d - Clinical trial sponsor’s GCP responsibilities
By Zenosis
The sponsor of a clinical trial takes responsibility for its initiation, management, and/or financing. A sponsor may transfer any or all of the sponsor’s trial-related duties and functions to a contract research organisation, but the ultimate responsibility for the quality and integrity of the trial data always resides with the sponsor. Duties and functions discussed in this short course include trial design, selection of investigators, QA and QC, data handling and record keeping, finance and compensation, regulatory submissions, management of investigational product(s), safety reporting, monitoring, audit, dealing with noncompliance, and clinical trial reports. ICH guideline E6 (revision 2) encourages sponsors to adopt a risk-based approach to managing the quality of trials. We discuss this approach in general, and aspects such as risk-based monitoring in particular.