- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7576 Medicine & Nursing courses near Hayes delivered Online
Sale Ends Today Level 2 Certificate in Understanding Nutrition and Health Admission Gifts FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter A person with great knowledge of Nutrition and Health has great value in the Health & Fitness industry. In the UK, the wellness industry is booming, with an estimated market value of £23 BILLION. The "Level 2 Certificate in Understanding Nutrition and Health Training" bundle is your gateway to a rewarding career in this thriving sector. This comprehensive course bundle will equip you with the essential skills and knowledge to excel and help you establish yourself as a wellness coach or a food safety expert. Start with the foundational Level 2 Certificate, and you will build a solid understanding of nutrition principles. Advanced courses in Diet and Meal Planning, Food Safety, and Hygiene will enhance your expertise. Specialised training on Mental Health, Immunity-boosting Foods, and specific diets like Keto and Vegan will prepare you for diverse roles in the nutrition field. Additional courses in Kitchen Management and Restaurant Management, Food Microbiology, and Geriatric Nutrition ensure you have a well-rounded education that meets industry standards. Courses Are Included In This Level 2 Certificate in Understanding Nutrition and Health Training: Course 01: Level 2 Certificate in Understanding Nutrition and Health Course 02: Nutritionist Course Level 4 Course 03: Nutrition Training - Advanced Diet & Meal Planning Course 04: Diet and Nutrition Diploma Course 05: Food and Mood: Improving Mental Health Through Diet and Nutrition Course 06: Diet Training for Weight Loss Course 07: Nutrition: 60+ Foods For Health, Fitness & Dieting Course 08: Immunity Boosting Food Course 09: Nutritional Therapy Course 10: HACCP Training Course 11: Kitchen Management Course 12: Restaurant Management Course 13: Catering - Catering Management Course 14: Supervising Food Safety in Catering Course 15: How to Improve Your Food Hygiene Rating Course 16: Food Hygiene Certificate Course 17: Food Hygiene Training Level 2 Course 18: Level 3 Food Allergen Awareness Course 19: Food Safety Training Certificate Course 20: Oven Cleaning Training Course Course 21: Food Microbiology Course 22: Paleo Diet Mastery Course 23: Ketogenic Diet Mastery: Achieving Health Goals with Keto Course 24: Fitness and Nutrition Fundamentals Course 25: Sports Nutrition Course 26: Vegan Nutritionist Course 27: Mastering Plant-Based Cooking Techniques Course 28: Understanding Mediterranean and Okinawa Diets Course 29: Food Preparation Mastery Course 30: Eating Disorder Awareness: Supporting Mental Health Elevate your health and nutrition sector career with the "Level 2 Certificate in Understanding Nutrition and Health Training" bundle. Let us help you by accepting our gift of learning with a Level 2 Certificate in Understanding Nutrition and Health course, and a high-flying job in a relevant field could soon be yours! Enrol today and start your journey towards becoming a leader in nutrition and health! Learning Outcomes of this Bundle: Gain a comprehensive understanding of nutrition and health principles. Master advanced diet and meal planning techniques for diverse needs. Learn to improve mental health through diet and nutrition. Develop skills in food safety, hygiene, and allergen awareness. Understand the impact of various diets, including keto and vegan. Enhance knowledge in kitchen and restaurant management. Why People Love Level 2 Certificate in Understanding Nutrition and Health Training From the Academy for Health & Fitness: Get a Free CPD Accredited Certificate upon completion of the course Get a Free Student ID Card with this training program (£10 postal charge will be applicable for international delivery) The course is Affordable and Simple to understand Get Lifetime Access to the course materials The training program comes with 24/7 Tutor Support Start your learning journey straight away! This diploma offers learners the opportunity to acquire a Certificate that is highly valued in the field of Nutrition and Health. With this Certification, graduates are better positioned to pursue career advancement and higher responsibilities within the Nutrition and Health setting. The skills and knowledge gained from this course will enable learners to make meaningful contributions to Nutrition and Health-related fields, impacting their Nutrition and Health experiences and long-term development. Course Curriculum Course 01: Level 2 Certificate in Understanding Nutrition and Health Module 01: Introduction to Nutrition Module 02: Nutrients for Life - Nutrients for Life Macronutrients Module 03: Nutrients for Life - Micronutrients Module 04: Nutrition Requirements from Pregnancy to Teenage Module 05: Nutrition Requirements for Adults and Various Groups Module 06: Healthy Dieting Module 07: Eating Disorders Module 08: Diet and Nutrition Related Diseases Module 09: Use of Nutrition in Different Illnesses/Diseases Module 10: Weight Control Management Module 11: Weight Loss Management Module 12: Basics of Ketogenic Diet Module 13: The Role of Nutritionist Course 02: Nutritionist Course Level 4 Module 01: Introduction to Basic Health Module 02: Overview of Nutritional Terms Module 03: Understanding Food Labels and Claims Module 04: The Science of Nutrition & Professionals Module 05: Carbohydrates - Sugar Module 06: Carbohydrates - Starch Module 07: Carbohydrates - Fiber Module 08: Protein Module 09: Fat - Omega 3, 6, 9 & Cholesterol Module 10: Minerals Starting With Iron & Zinc Module 11: More Minerals - Calcium, Phosphorus, Magnesium Module 12: Vitamins - Starting with B's Module 13: Vitamins A, C, E, K - the Antioxidants Module 14: Couple More Minerals - Sodium & Potassium Module 15: Trace Minerals, B12 & Vitamin D Module 16: Nutritional Deficiencies & Food Allergies Module 17: Organic vs Toxic Chemicals - Finding Balance Module 18: Fads, Trends, and Extremes in Diet Module 19: Exercise Unlocks Nutrients Module 20: Water Module 21: Rest Module 22: Calorie Intake & Menu Development Course 03: Nutrition Training - Advanced Diet & Meal Planning Section 01: Introduction Section 02: The Nutrition Fundamentals Section 03: Advanced Dieting Theory Section 04: Advanced Dieting For Fat Loss Section 05: Advanced Dieting For Muscle Growth Section 06: Popular Diets Explained Section 07: Dieting & Disease Prevention =========>>>>> And 27 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*30 = £390) CPD Hard Copy Certificate: Free (For The First Course: Previously it was £29.99) CPD 300 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Aspiring nutritionists Wellness coaches Food safety professionals Healthcare providers Diet planners Restaurant managers Requirements You will not need any prior background or expertise to enrol in this course. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Nutritionist: £25,000 - £45,000 Wellness Coach: £20,000 - £40,000 Food Safety Officer: £22,000 - £35,000 Dietitian: £24,000 - £45,000 Restaurant Manager: £25,000 - £50,000 Health Coach: £20,000 - £40,000 Certificates CPD Accredited Digital certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. CPD Accredited e-Certificate - Free Enrolment Letter - Free Student ID Card - Free CPD Accredited Hard copy certificate Hard copy certificate - Included Upon completion of the course, you can order a Free Hard Copy Certificate Accredited by CPD QS, accepted throughout the UK and internationally. If you are an international student, then you have to pay an additional 10 GBP for each certificate as an international delivery charge.

CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT03e - Clinical trial investigator’s GCP responsibilities
By Zenosis
A clinical investigator is responsible for conducting the clinical trial in compliance with the study protocol, GCP, medical ethics, and applicable legal requirements. The clinical research community expects that investigators and clinical staff are fully trained in GCP. Duties and functions discussed in this short course include: provision of adequate resources; liaison with IRB/IEC; compliance with protocol; management of investigational product(s), informed consent and data records; and safety reporting.
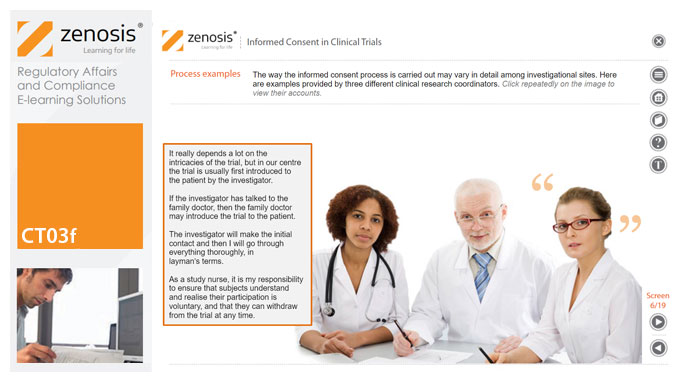
CT03f - Informed consent in clinical trials
By Zenosis
Informed consent in clinical research is an ethical and regulatory requirement. A research subject must enter a study voluntarily, be informed about risks and benefits, and understand the difference between investigation and treatment. Subjects must not be coerced into enrolment, nor must they be enticed by exaggerated claims of benefit. Before they can enrol, all potential subjects must agree, in writing, to participate. In addition to ethical and regulatory imperatives, the potential for litigation by subjects further highlights the importance of rigorous adherence to informed consent principles. In this short course we set out the principles and requirements and provide examples of practical issues confronting healthcare professionals and subjects.
CT04b - Clinical protocol design
By Zenosis
Clinical trial protocols are an essential part of clinical trial design. Protocol documents are critical to conducting safe and cost-effective investigations. Protocol documents are large and complex, containing comprehensive information relating to purpose, design and conduct of a clinical trial. Aspects of a protocol include patient eligibility criteria, and treatment specifications. This short course provides an overview of clinical trial protocols. Opportunities to improve a clinical trial protocol for regulatory approval are also discussed.
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
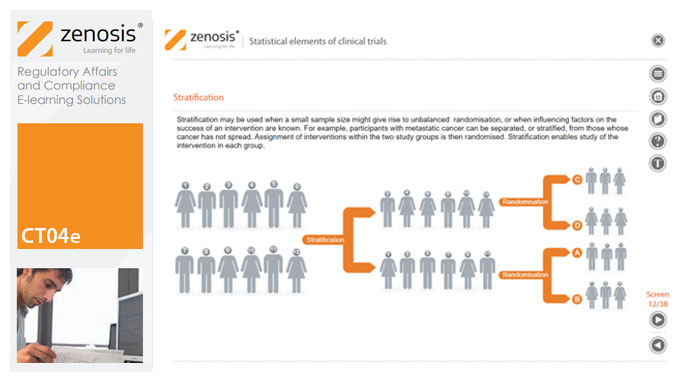
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.