- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
7445 Medicine & Nursing courses in Walkden delivered Online
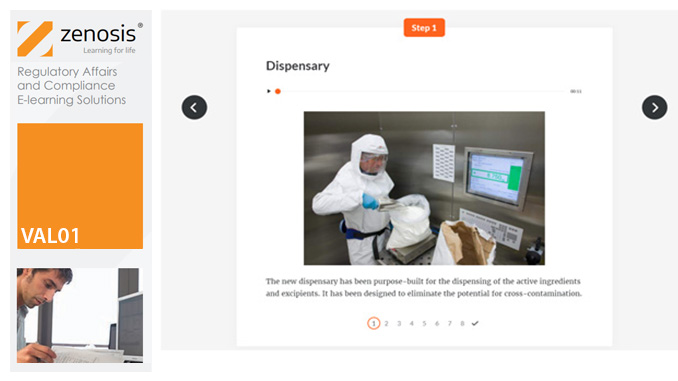
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
VAL04: Operational and Performance Qualification
By Zenosis
Having undergone Installation Qualification, before equipment can be used routinely in production, it needs to undergo Operational Qualification (OQ) and Performance Qualification (PQ). This module describes OQ and PQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

FAA Level 1 Award In Awareness Of Safeguarding (RQF) Face-to-Face Classroom: Half-day course Virtual Classroom: 2 sessions of 2 ½ hours For those who work with children, young people and vulnerable adults Promotes awareness of safeguarding, enabling learners to identify problems and show where to report these to Course Contents: Safeguarding legislation and guidance Key safeguarding roles Different types of abuse Signs and indicators of abuse and neglect Actions to take when a safeguarding concern arises Benefits of this course: In 2018/2019, 415,050 concerns of abuse were raised In 2018/2019, there were nearly 400,000 children in need 52,300 children were subject to a child protection plan 63% of adult safeguarding concerns are for people over 65 1 in every 42 adults aged 85+ have required safeguarding enquires... Child abuse often goes unreported and unrecorded - till it is picked up on by someone who then does something about it. This Level 1 Safeguarding Awareness course gives people the knowledge to make a real difference to a person's life! This basic Safeguarding course is a nationally recognised, Ofqual regulated qualifications accredited by First Aid Awards Ltd. This means that you can be rest assured that your Level 1 in Safeguarding Certificate provides information for best practice to make a real difference to protect the health and wellbeing of the most vulnerable in our society. The Ofqual Register number for this course is 603/5635/2

Policies. Legislation. CQC Medication. Routes. Formulation. Types. Absorption. About this event Policies. Legislation. CQC Medication. Routes. Formulation. Types. Absorption. MDT. Roles and Responsibilities. Levels of support. Boundaries and agreed ways of working. Order. Receive. Store. Record. Transfer. Dispose. 7 R’s. Sources of info. Practical session including MAR charts. Risk assessments. Person Centred Care. Review. Covert. Consent. Refuse. Errors and action. Infection Control and Technique.

SUB03: The Mutual Recognition Procedure (MRP)
By Zenosis
his module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the MRP.
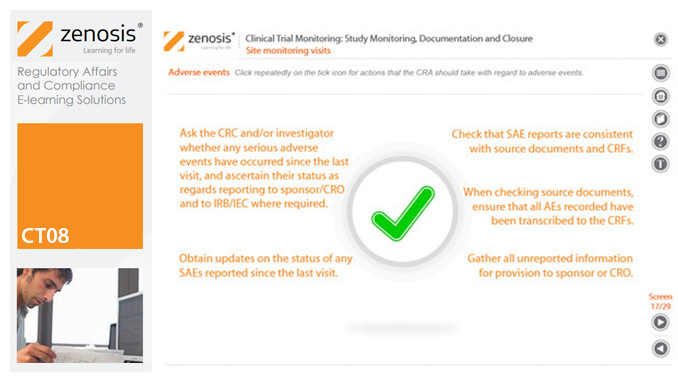
CT08: Clinical Trial Monitoring: Study Monitoring, Documentation and Closure
By Zenosis
The sponsor of a clinical trial must arrange for it to be monitored throughout its duration to ensure that the rights and wellbeing of subjects are protected, the trial data are accurate, complete and verified from source documents, and the conduct of the trial complies with the study protocol, Good Clinical Practice and regulatory requirements. In this module we describe how a Clinical Research Associate (CRA) monitors an ongoing trial to its conclusion.
SUB11: The Decentralised Procedure (DCP)
By Zenosis
This module describes the roles of the various players in the procedure, the sequence and duration of the stages involved, and the requirements on content, format and timing of submissions. It discusses the special issues that apply to generic products in the DCP.