- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1661 Management courses in Ewell
Assessing the Water-Steam Chemistry Cycle in Thermal Power Plants
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) This 2-half-day Virtual Instructor Led Training (VILT) course will discuss the chemical aspects of the water-steam cycle in a power plant. The VILT course will examine the different types of chemicals used in boilers, potential issues in a water-steam cycle as well as aspects of monitoring and specifications regarding target values and alarm levels. Participants will also be equipped on what to do or key action steps to take in the event of chemistry-related incidents. This course is delivered in partnership with ENGIE Laborelec. Training Objectives The VILT course will cover the following: Detailed aspects of chemistry in a water steam cycle, including types of chemicals used in boilers depending on the treatment and type of boiler Potential issues in a water-steam cycle such as corrosion and deposition Monitoring & analytical programmes and knowledge of specifications for the water steam cycle (normal values targets - alarm levels) Chemistry aspects during transition periods: start-up, shutdown and preservation Actions to be taken in the event of an alarm Examples of incidents or deviations compared to normal chemistry Target Audience The VILT course is intended for: Power plant chemists Plant operation or maintenance engineers Consultants and technical project managers Boiler engineers Course Level Basic or Foundation Training Methods The VILT course will be delivered online in 2 half-day sessions comprising 4 hours per day, with 2 x 10 minutes break per day, including time for lectures, discussion, quizzes and short classroom exercises. Course Duration: 2 half-day sessions, 4 hours per session (8 hours in total). Trainer Your expert course leader is a chemistry consultant in the energy sector. He works with operators of power plants and industrial facilities. He is active in water-steam cycle chemistry, where he provides support to increase chemistry maturity through audits, trainings or development of key performance indicators. His role also includes operational assistance in the field of chemical cleaning and troubleshooting. More recently, he expanded his field of competence towards electrical storage. In this regard, he specializes in electrochemistry and is in charge of different tests on batteries and their components within the ENGIE Batteries Lab. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

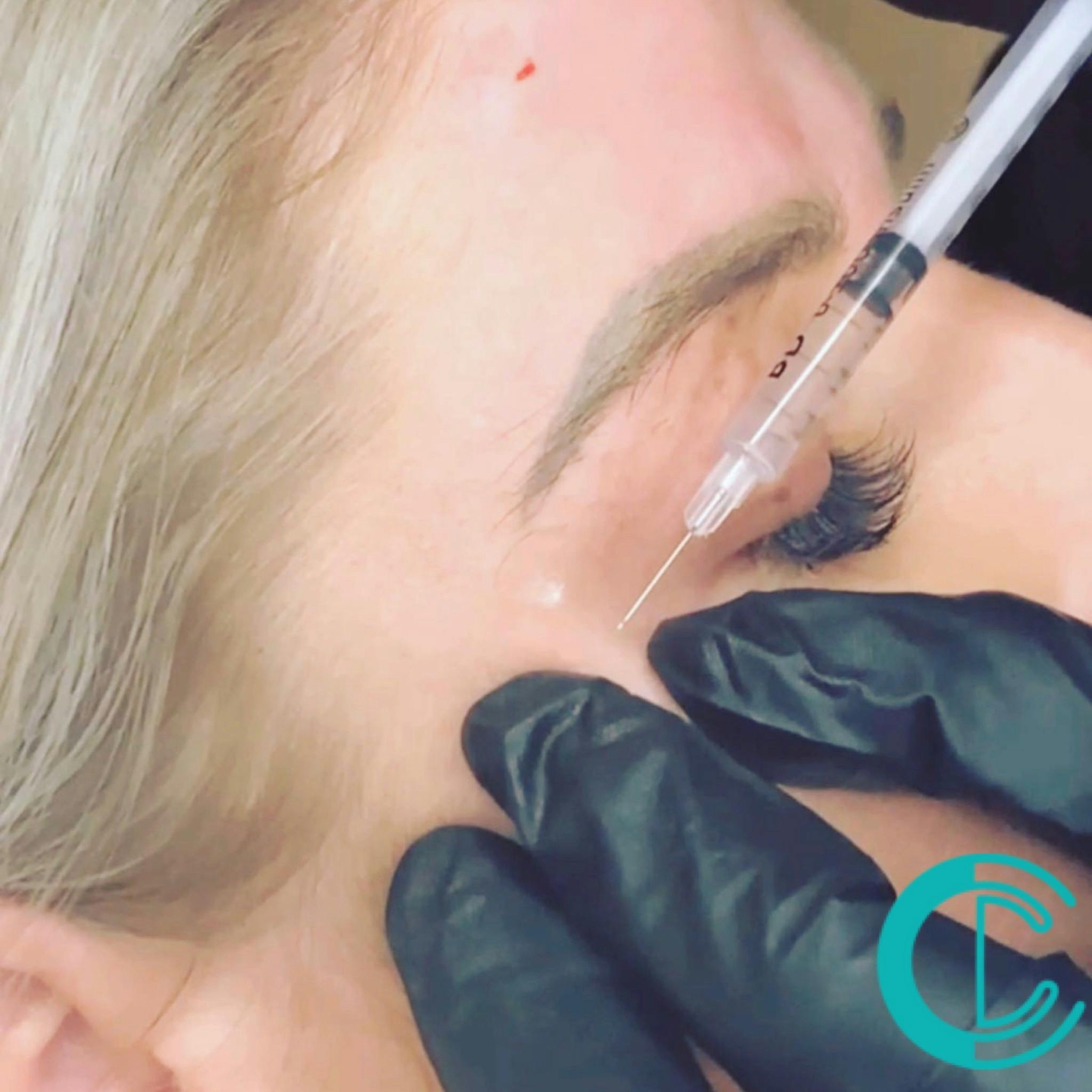
Anti Wrinkle Injections Training
By Cosmetic College
Our students gain the product knowledge and practical skills needed to perform foundation anti-wrinkle injections to the upper third of the face. You will learn the fundamentals of facial anatomy, muscle structure, and the ageing process. By understanding these key concepts, you will be able to assess your client's needs accurately and create customised treatment plans to achieve optimal results. Through hands-on practical sessions, you will gain the confidence and proficiency to administer anti-wrinkle injections with precision. Our experienced trainers will guide you through various injection techniques, dosage calculations, and proper injection site selection. Patient safety is our top priority, and you will learn important safety protocols to minimise risks and manage potential complications. By implementing best practices, you will ensure a comfortable and secure experience for your clients. Course Entry Requirements: One or more of the following: Be a medical professional registered to a medical body (NMC, GMC, GDC, GPhC, etc.) Have Level 3 NVQ in Beauty Therapy. Have six months of experience in SPMU, Microblading, and Microneedling) and six months of Anatomy & Physiology Level 3. Have 12 months of experience in advanced beauty treatments (e.g SPMU, Microblading, Microneedling). Course Pre-Study/Practical & Length: Pre Study 1-day on-site training Course Agenda: Product History Face anatomy Injection techniques Ageing process Patient consultation & expectations Tutor demonstration Student practical session Delivery method Areas Glabella lines, Cross feet, Frown lines Course Benefits Student Benefits Enhanced Knowledge and Skills: You will gain in-depth knowledge of facial anatomy, muscle structure, and the ageing process, enabling you to accurately assess clients' needs and develop personalised treatment plans. Through practical training, you will acquire the skills and confidence to administer anti-wrinkle injections with precision. Professional Credibility: Completing this course will establish you as a qualified and competent practitioner in the field of anti-wrinkle injections. Your certification will enhance your professional credibility and increase your chances of securing employment in reputable medical spas, aesthetic clinics, or starting your own practice. Expanded Career Opportunities: The aesthetics industry is continuously growing, and there is a high demand for skilled professionals in the field of anti-wrinkle treatments. By enrolling in this course, you will position yourself for exciting career opportunities and the potential for career advancement. Client Benefits Personalised Treatment Plans: With your enhanced knowledge and skills, you will be able to assess clients' unique facial features, concerns, and expectations. This will enable you to develop customised treatment plans tailored to their specific needs, ensuring optimal results. Safe and Effective Treatments: The comprehensive training you receive will prioritise patient safety and risk management. You will learn proper injection techniques, dosage calculations, and safety protocols, minimising risks and ensuring a safe and comfortable experience for clients. Youthful and Refreshed Appearance: By administering anti-wrinkle injections, you can help clients achieve a smoother, more youthful appearance. By reducing the appearance of wrinkles and fine lines, clients will experience increased confidence and satisfaction with their rejuvenated appearance. Earning Potential By completing this course, you can unlock significant earning potential. As a certified practitioner in anti-wrinkle injections, you can expect competitive salaries and income opportunities in the aesthetics industry. The exact earning potential may vary depending on factors such as your location, experience, and clientele. With the growing demand for anti-wrinkle treatments, you have the opportunity to build a successful career and secure a rewarding salary. Whether you choose to work in established clinics, medical spas, or start your own practice, the ability to offer anti-wrinkle injections can significantly enhance your earning potential. Enrol in our Foundation Anti Wrinkle Injections Training Course today and take the first step towards a lucrative career in aesthetics. Unlock your earning potential and make a positive impact in the lives of your clients. Frequently Asked Questions What topics are covered in the course curriculum? Our course curriculum covers essential topics such as facial anatomy, injection techniques, product selection, client consultation, and post-treatment care. You will receive comprehensive theoretical knowledge and hands-on practical training to ensure a well-rounded learning experience. Do I need to bring my own models for the practical training? No, it is not necessary to bring your own models for the practical training sessions. We provide models for you to practice on under the guidance of our experienced instructors. However, if you prefer to bring your own models, you are welcome to do so. Will I receive a certification upon course completion? Yes, upon successfully completing the course, you will receive a certification that recognises your proficiency in performing anti-wrinkle injections. This certification will enhance your professional credibility and open doors to career opportunities.
Microsoft Project Advanced - In-company (now with trainer led live online classes)
By Microsoft Office Training
Course Objectives At the end of this course you will be able to: Manage project costs Consolidate and reuse project plan information Exchange project plan data with other applications Update a project plan Customise Project to meet specific requirements ' 1 year email support service Take a look at the consistent excellent feedback from our corporate clients visiting our site ms-officetraining co uk Customer Feedback Really useful and engaging course. Learnt a lot that will be very beneficial in my job. Trainer was great. Kelly Moreley - TACT Very happy with the course. Worked as a good refresher from what I knew already and enhanced my knowledge further Jenny Price - Acer ' With more than 20 years experience, we deliver courses on all levels of the Desktop version of Microsoft Office and Office 365; ranging from Beginner, Intermediate, Advanced to the VBA level. Our trainers are Microsoft certified professionals with a proven track record with several years experience in delivering public, one to one, tailored and bespoke courses. Our competitive rates start from £550.00 per day of training Tailored training courses: You can choose to run the course exactly as they are outlined by us or we can customise it so that it meets your specific needs. A tailored or bespoke course will follow the standard outline but may be adapted to your specific organisational needs. Exchanging Project Plan Data with Other Applications Import a list of tasks from Outlook Import a Task List from an Excel File Create a Custom Import Map Export Project Plan Cost Data into Excel Save Project Plan Information as a Web Page Updating a Project Plan Enter Task Progress Information View Task Progress Split a Task Reschedule a Task Creating Custom Fields Text fields Lookup fields Calculated fields with the use of formulas and functions Inserting Graphical Indicators Customizing how you look at data Filter Tasks in a Project Plan Create a Custom Table Create a Custom View Copy custom Views and Tables between projects Earned value analysis Understanding Earned Value Fields View Multiple Baselines in a Single Project Tracking progress between interim plans Creating Custom Reports Create a Custom Report Modify a Custom Report's Header and Footer Add a Picture to a Report Modify a Custom Report's Margins Print a Custom Report Re-using Project Plan Information Create a Project Plan Template Create a Custom Combination View Make Custom Views Available to Other Project Plans Share Resources between Projects, Create a Master Project Plan with sub projects Who is this course for? Who is this course for? This course is designed for those that who already have the skills to create and modify project plans and would like to acquire a more in depth and thorough knowledge of more advanced functionalities in project planning and tracking. Requirements Requirements Preferably, delegates would have attended the MS Project Introduction course. Career path Career path Microsoft Office know-how can instantly increase your job prospects as well as your salary. 80 percent of job openings require spreadsheet and word-processing software skills

Disciplined Agile Senior Scrum Master (DASSM): In-House Training
By IIL Europe Ltd
Disciplined Agile Senior Scrum Master (DASSM): In-House Training Do you want to take Disciplined Agile® to a new level? Are you looking for tools to solve complex problems and enhance your organization's agility? Do you want to learn how to lead your team to excellence? Expand your knowledge and build practical skills around Disciplined Agile®, business agility, leadership, and team development. Disciplined Agile Senior Scrum Master is a nine-lesson, instructor-led course that shows you how to use the Disciplined Agile tool kit to solve a variety of advanced problems, work with allies within your organization, and optimize how teams work. You will gain knowledge in planning, reporting and metrics, and coordinating activities, as well as how to meet challenges in these areas. And you'll develop the skills you need to foster emotional intelligence, resolve conflicts, and lead high-performance teams at any stage of development. Filled with activities, supplemental reading, and more, this course will prepare you to take the Disciplined Agile Senior Scrum Master (DASSM) exam and, equally important, start using Disciplined Agile immediately within your leadership role. What You Will Learn After the completion of this course, you will be able to: Accelerate your ability to lead high-profile initiatives that are critical to enterprise success Take a deep dive into the Disciplined Agile® tool kit to develop a comprehensive understanding of the hundreds of practices and strategies it contains and the trade-offs of applying them Apply the Disciplined Agile tool kit in hands-on exercises to guide your team in choosing and evolving your best way of working (WoW) in real-life situations Use the tool kit to solve complex challenges commonly encountered in development and operational teams, the value stream, and at the enterprise level Learn how to design and implement metrics that measure your improvements in areas where your teams are struggling Understand how to nurture emotional intelligence Feel confident empowering others on your team(s) Learn how to lead your teams in any situation to improve value delivery for your customers Apply the Disciplined Agile tool kit to guide your team in choosing and evolving the best way of working (WoW) in the situation you face Be prepared to take the Disciplined Agile® Senior Scrum Master (DASSM) exam and earn a valuable, credible certification Roles and responsibilities of DASSM Tuckman Team Development Model Emotional intelligence and why it is essential to team performance Business agility Tactical scaling factors in more complex situations Disciplined DevOps layer 'Test-first' method as it relates to the quality of requirements Scope and purpose of the value stream layer Coordinate activities process goal and why it is important Value creation structure of teams DA™ tool kit to optimize the flow of work and solve challenges related to coordinating and collaborating across teams, or within a larger team of teams Thomas-Kilmann Conflict Resolution Planning Five levels of scope Metrics

Disciplined Agile Senior Scrum Master (DASSM)
By IIL Europe Ltd
Disciplined Agile Senior Scrum Master (DASSM) Do you want to take Disciplined Agile® to a new level? Are you looking for tools to solve complex problems and enhance your organization's agility? Do you want to learn how to lead your team to excellence? Expand your knowledge and build practical skills around Disciplined Agile®, business agility, leadership, and team development. Disciplined Agile Senior Scrum Master is a nine-lesson, instructor-led course that shows you how to use the Disciplined Agile tool kit to solve a variety of advanced problems, work with allies within your organization, and optimize how teams work. You will gain knowledge in planning, reporting and metrics, and coordinating activities, as well as how to meet challenges in these areas. And you'll develop the skills you need to foster emotional intelligence, resolve conflicts, and lead high-performance teams at any stage of development. Filled with activities, supplemental reading, and more, this course will prepare you to take the Disciplined Agile Senior Scrum Master (DASSM) exam and, equally important, start using Disciplined Agile immediately within your leadership role. What You Will Learn After the completion of this course, you will be able to: Accelerate your ability to lead high-profile initiatives that are critical to enterprise success Take a deep dive into the Disciplined Agile® tool kit to develop a comprehensive understanding of the hundreds of practices and strategies it contains and the trade-offs of applying them Apply the Disciplined Agile tool kit in hands-on exercises to guide your team in choosing and evolving your best way of working (WoW) in real-life situations Use the tool kit to solve complex challenges commonly encountered in development and operational teams, the value stream, and at the enterprise level Learn how to design and implement metrics that measure your improvements in areas where your teams are struggling Understand how to nurture emotional intelligence Feel confident empowering others on your team(s) Learn how to lead your teams in any situation to improve value delivery for your customers Apply the Disciplined Agile tool kit to guide your team in choosing and evolving the best way of working (WoW) in the situation you face Be prepared to take the Disciplined Agile® Senior Scrum Master (DASSM) exam and earn a valuable, credible certification Roles and responsibilities of DASSM Tuckman Team Development Model Emotional intelligence and why it is essential to team performance Business agility Tactical scaling factors in more complex situations Disciplined DevOps layer 'Test-first' method as it relates to the quality of requirements Scope and purpose of the value stream layer Coordinate activities process goal and why it is important Value creation structure of teams DA™ tool kit to optimize the flow of work and solve challenges related to coordinating and collaborating across teams, or within a larger team of teams Thomas-Kilmann Conflict Resolution Planning Five levels of scope Metrics

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

Practical Approach to Auditing Systems and Processes
By Research Quality Association
Course Information Our extensively proven course delves into the essential stages of process and system auditing. Gain invaluable insights and direction in auditing systems and processes, spanning across global and local organisational levels. This course will assist delegates with: A practical approach for the development and conduct of process and system audits An enhanced understanding of key system audit principles, preparation, design and conduct Increased expertise, efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Share knowledge and experiences. By the end of the course delegates will be better able to: Design and plan more effectively to achieve their process and systems audit objectives and add value to their organisation Improve the effectiveness, focus and credibility of the audit programme Understand the key system audit principles, preparation, design and conduct Develop system audit tools to ensure more effective audit conduct and outcome Create audit strategies utilising risk management principles Prepare for inspections. Tutors Tutors will be comprised of (click the photos for biographies): Allison Jack Executive Director, Bristol Myers Squibb Rocio Castellanos Director, Pfizer Ltd Guy Houben G(C)LP Auditor, Janssen Pharmaceutical Companies of Johnson & Johnson Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introductions, Expectations/Challenges/Experiences A discussion to explore the range of approaches to the conduct of systems audit. 09:30 Introducing Systems Audit What is a system? Why conduct system audits? Advantages, disadvantages and challenges. 10:20 Break 10:35 Systems Audit Design and Planning Identifying the customer, setting objectives, development of the audit plan and audit tools, plans for the audit report. 12:00 Designing System Audit Tools 12:45 Lunch 13:30 System Audit Plan - Exercise 14:00 Introduction to Case Studies The objectives of the case studies are defined and process and outputs described. 14:15 Case Studies - Session 1 A first opportunity for work on case studies. Defining objectives and scope and understanding the requirements of the audit client. 15:00 Break 15:20 Case Studies - Session 1 continued 16:30 Case Studies - Feedback 17:00 Close of Day 1 Day 2 09:00 Simple System Audit Example - Introduction The objectives of the case studies are defined and process and outputs described. 09:10 Case Studies - Session 2 - A Simple System Audit Example An example of system audit applied to a simple system. 10:30 Break 10:45 A Simple System Audit Example - Case Study Feedback 11:30 Strategy Audit programme planning. 12:15 Lunch 13:00 Case Studies - Session 3 Work on delegate's case studies. 14:30 Break 14:45 Case Studies - Session 3 - Feedback 15:15 Closing remarks 15:30 Close of course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Search By Location
- Management Courses in London
- Management Courses in Birmingham
- Management Courses in Glasgow
- Management Courses in Liverpool
- Management Courses in Bristol
- Management Courses in Manchester
- Management Courses in Sheffield
- Management Courses in Leeds
- Management Courses in Edinburgh
- Management Courses in Leicester
- Management Courses in Coventry
- Management Courses in Bradford
- Management Courses in Cardiff
- Management Courses in Belfast
- Management Courses in Nottingham