- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3562 Courses in Swanley
Fundamentals of E&P Data Management
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) The energy industry has started its journey to be more data centric by embracing the industry 4.0 concept. As a result, data management - which was considered until recently as a back-office service to support geoscience, reservoir management, engineering, production and maintenance - is now given the spotlight! To become an active stakeholder in this important transition in E&P data management, it is necessary to understand the new technical opportunities offered by the Cloud, Artificial Intelligence and how data governance can pave the way towards more reliable and resilient processes within E&P domain. Several key questions that need to be addressed: Why place more focus on data assets? Is data management just about serving geoscientists or engineers with fresh data? What is the value of data management in the E&P sector for decision making? How to convince the data consumers that the data we provide is reliable? Is the data architecture of my organization appropriate and sustainable? The purpose of this 5 half-day Virtual Instructor Led Training (VILT) course is to present the data challenges facing the energy organizations today and see how they practically solve them. The backbone of this course is based on the DAMA Book of Knowledge for Data Management. The main data management activities are described in sequence with a particular focus on recent technological developments. Training Objectives Upon completion of this VILT course, the participants will be able to: Understand why the data asset is now considered as a main asset by energy organizations Appreciate the importance of data governance and become an active stakeholder of it Understand the architecture and implementation of data structure in their professional environment Get familiarized with the more important data management activities such as data security and data quality Integrate their subsurface and surface engineering skills with the data managements concepts This VILT course is unique on several points: All notions are explained by some short presentations. For each of them, a set of video, exercises, quizzes will be provided to help develop an engaging experience between the trainer and the participants A pre-course questionnaire to help the trainer focus on the participants' needs and learning objectives A detailed reference manual A lexicon of terms for data-management Limited class size to encourage the interactivity Target Audience This VILT course is intended for: Junior/new data managers Geoscientists Reservoir engineers Producers Maintenance specialists Construction specialists Human resources Legal Course Level Basic or Foundation Training Methods The VILT course will be delivered online in 5 half-days consisting 4 hours per day, with 2 breaks of 10 minutes per day. Course Duration: 5 half-day sessions, 4 hours per session (20 hours in total). Trainer Your expert course leader is a geologist by education who has dedicated his career to subsurface data management services. In 2016, he initiated a tech startup dedicated to Data Management using Artificial Intelligence (AI) tools. He is heavily involved in developing business plans, pricing strategies, partnerships, marketing and SEO, and is the co-author of several Machine Learning publications. He also delivers training on Data Management and Data Science to students and professionals. Based in France, he was formerly Vice President, Sales & Marketing at CGG where he was in charge of the Data Management Services strategy, Sales Manager at Spie O&G Services where he initiated the Geoscience technical assistance activities and Product Manager of interactive seismic inversion software design and marketing at Paradigm. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

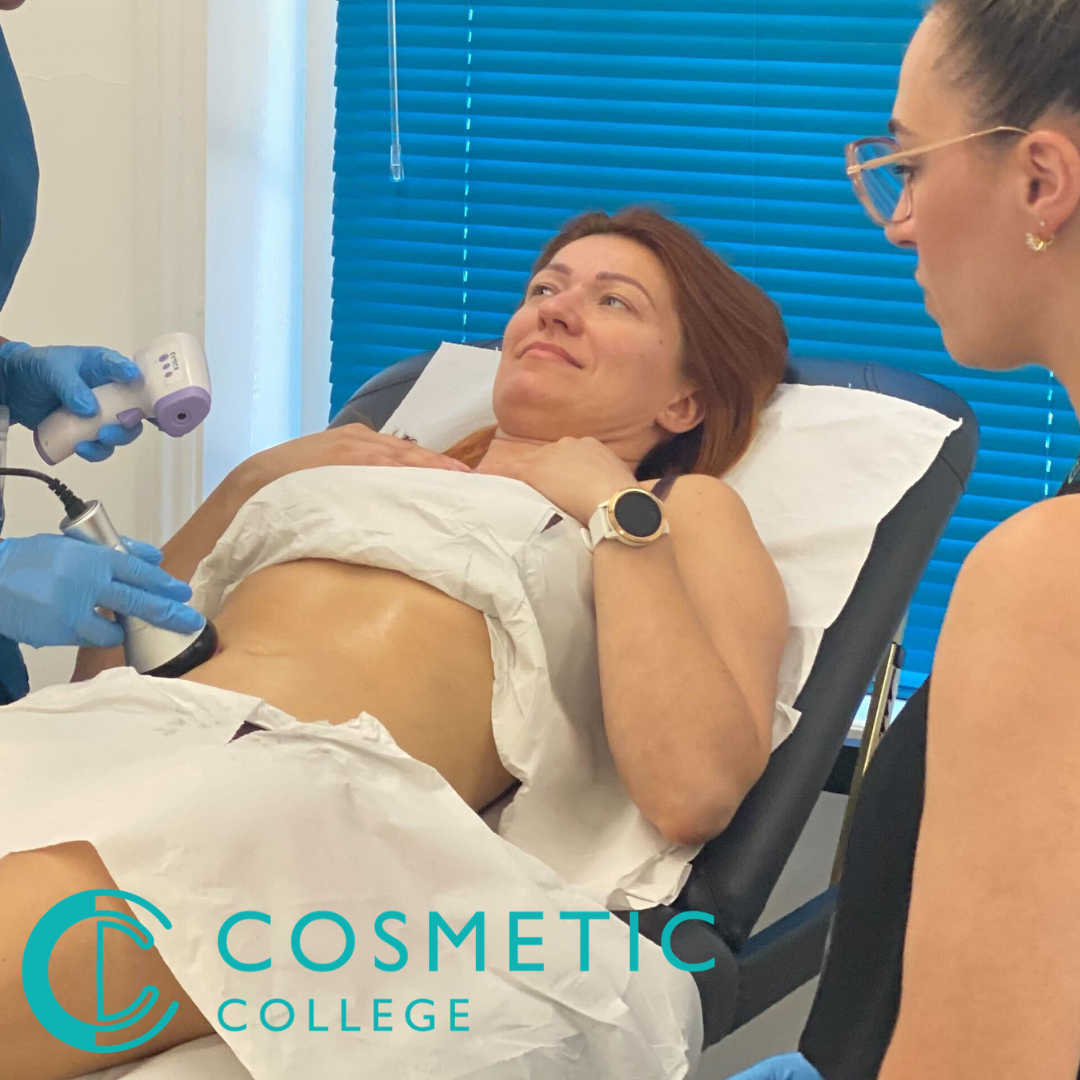
Body Contouring Training Package
By Cosmetic College
This course is designed to teach students how to safely and successfully carry out a variety of body contouring treatments at the best value available in the market. Our course offers a blend of practical training and online learning to give you the knowledge and skills to deliver face and body waxing services to your clients. Our courses are kept intimate with a maximum of 6 learners to a class. Courses Included Wood Therapy Lymphatic Drainage Massage Fat Freezing Ultrasound Cavitation Radio Frequency Skin Tightening This package is delivered in a combined format with e-learning provided to be completed ahead of an intense 4 day practical training days.
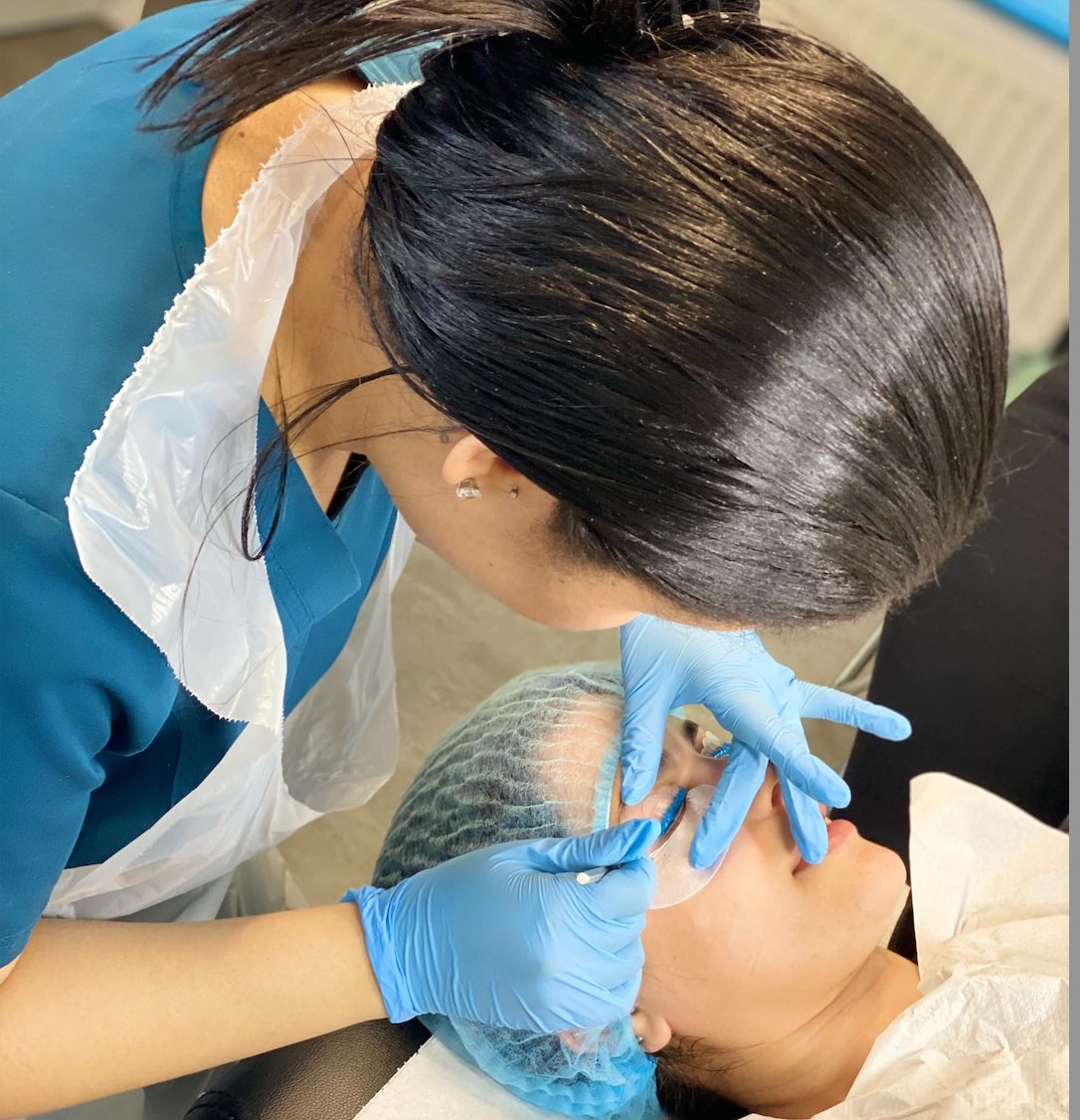
Lash & Brow Business Training Package
By Cosmetic College
Our lash and brow package includes 5 courses designed for students that would like to start a business in the beauty industry as eyebrow and eyelash technicians. After completing these courses, students will have the practical skills, competency, and business knowledge to deliver professional treatments and exceptional client care with a business plan. Our tutors are experienced expert practitioners and educators who own their own salons and clinics and have worked at some of the world's most prestigious beauty clinics and spas. Our education team has collectively over 70 years of experience providing safe, effective treatments, running successful businesses, and have taught hundreds of students; rest assured that you will be leaning on tutors with a wealth of knowledge to help you make this next new exciting step to start your own beauty and aesthetics business. Beauty Courses Included Russian Eyelash Extensions Classic Eyelash Extensions High Definition Brows Infection Control (Online) Brow Lamination Lash Lift First aid (Online) Our students are given 12 months to complete all the courses in this package. Starter kits are not included in the package but are available to purchase at a discounted rate for package students.
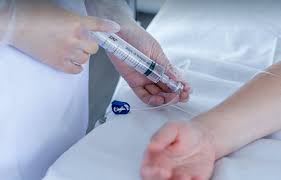
Intravenous Administration of Fluid and Medication
By Guardian Angels Training
Gain the knowledge and skills for safe and effective intravenous therapy with our "Intravenous Administration of Fluid and Medication" course. Ideal for healthcare professionals administering IV fluids and medications.
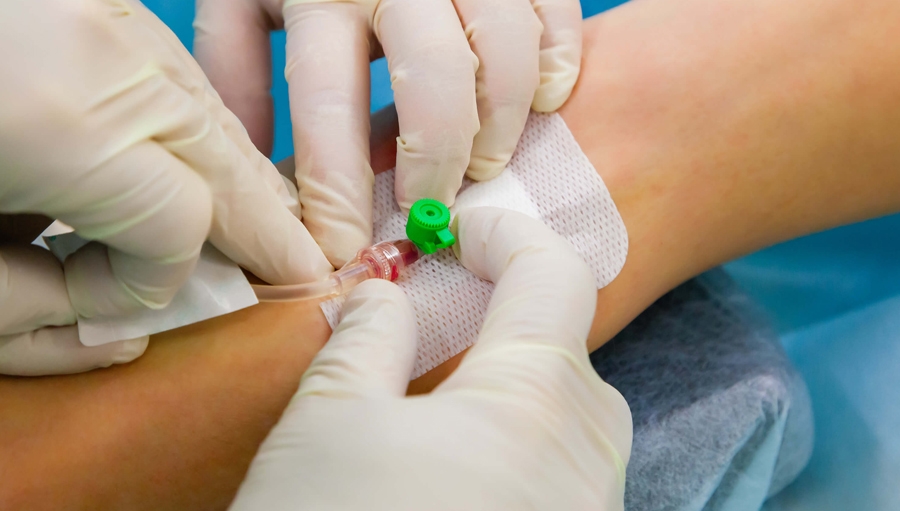
Peripheral Intravenous Cannulation (Peripheral vascular access device)
By Guardian Angels Training
Gain the knowledge and skills to safely insert and manage peripheral IV cannulas with our training course for healthcare professionals. Ideal for nurses, medical personnel, and other practitioners.
Level 3 Endorsed Award in Delivering Health and Social Care Training (Healthcare Train the Trainer)
By Guardian Angels Training
Gain expertise in healthcare training with our Level 3 Endorsed Award in Delivering Health and Social Care Training. Our comprehensive program equips you with the skills and knowledge to become a proficient trainer in the healthcare sector.

Relay Protection in Power Systems
By EnergyEdge - Training for a Sustainable Energy Future
Elevate your understanding of relay protection in power systems with EnergyEdge's specialized classroom training course and gain valuable insights.

Cross Border Electricity Trading in Asia - Renewable Energy, Digital Technologies and New Operational Flexibility Solutions
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) This 3 half-day Virtual Instructor Led Training (VILT) course highlights the impact of the introduction of renewable energy, digital technologies and new operational flexibility solutions in the electricity trading market. These advancements facilitate unique opportunities and challenges for cross border trading of electricity. Most countries in Asia, have designed their own portfolio of climate actions with an accelerated penetration of renewable energy (or by importing renewable energy into their local grids). These changes are taking place at unprecedented speed and add further complexity to the operation of electricity trading markets, while presenting new opportunities. The Asian market, can tap into its vast potential of solar, wind and geothermal energy sources. A global, unified vision is emerging to support each of countries' energy needs and decarbonization goals. This VILT course will highlight priorities of each country to achieve its energy goals. The main operational flexibilities of each type of renewable resource are discussed in detail. The course also discusses the main components of Power Purchase Agreements and advancements in digitalization and how digital technologies can influence the energy market and cross border electricity trading. Key Takeaways: New Energy Market Design Cross Border Trading PPAs Mechanisms and Examples of PPAs The Increased Penetration of Renewable Resources in the Power Systems and How It Stimulates Cross Border Trading How Digital Innovation Drives Energy Markets and Cross Border Trading Training Objectives Upon completion of this VILT course, participants will be able to: Be familiar with the global vision of One Sun, One World, One Grid Understand the major trends reshaping the energy markets Learn how innovative digital technologies change the energy markets Understand why sustainable energy markets require a tighter coordination between transmission and distribution system operators Engage with each other to design the energy market of the future Target Audience This VILT course will benefit policy makers and regulators from energy agencies, transmission companies and utilities as well as power system engineers and power system operators from control centre and ISO. Training Methods The VILT will be delivered online in 3 half-day sessions comprising 4 hours per day, including time for lectures, discussion, quizzes and short classroom exercises. Course Duration: 3 half-day sessions, 4 hours per session (12 hours in total). Trainer Your expert course leader is a Utility Executive with extensive global experience in power system operation and planning, energy markets, enterprise risk and regulatory oversight. She consults on energy markets integrating renewable resources from planning to operation. She led complex projects in operations and conducted long term planning studies to support planning and operational reliability standards. Specializing in Smart Grids, Operational flexibilities, Renewable generation, Reliability, Financial Engineering, Energy Markets and Power System Integration, she was recently engaged by the Inter-American Development Bank/MHI in Guyana. She was the Operations Expert in the regulatory assessment in Oman. She is a registered member of the Professional Engineers of Ontario, Canada. She is also a contributing member to the IEEE Standards Association, WG Blockchain P2418.5. With over 25 years with Ontario Power Generation (Revenue $1.2 Billion CAD, I/S 16 GW), she served as Canadian representative in CIGRE, committee member in NSERC (Natural Sciences and Engineering Research Council of Canada), and Senior Member IEEE and Elsevier since the 90ties. Our key expert chaired international conferences, lectured on several continents, published a book on Reliability and Security of Nuclear Power Plants, contributed to IEEE and PMAPS and published in the Ontario Journal for Public Policy, Canada. She delivered seminars organized by the Power Engineering Society, IEEE plus seminars to power companies worldwide, including Oman, Thailand, Saudi Arabia, Malaysia, Indonesia, Portugal, South Africa, Japan, Romania, and Guyana. Our Key expert delivered over 60 specialized seminars to executives and engineers from Canada, Europe, South and North America, Middle East, South East Asia and Japan. Few examples are: Modern Power System in Digital Utilities - The Energy Commission, Malaysia and utilities in the Middle East, GCCIA, June 2020 Assessment of OETC Control Centre, Oman, December 2019 Demand Side management, Load Forecasting in a Smart Grid, Oman, 2019 Renewable Resources in a Smart Grid (Malaysia, Thailand, Indonesia, GCCIA, Saudi Arabia) The Modern Power System: Impact of the Power Electronics on the Power System The Digital Utility, AI and Blockchain Smart Grid and Reliability of Distribution Systems, Cyme, Montreal, Canada Economic Dispatch in the context of an Energy Market (TNB, Sarawak Energy, Malaysia) Energy Markets, Risk Assessment and Financial Management, PES, IEEE: Chicago, San Francisco, New York, Portugal, South Africa, Japan. Provided training at CEO and CRO level. Enterprise Risk methodology, EDP, Portugal Energy Markets: Saudi Electricity Company, Tenaga National Berhad, Malaysia Reliability Centre Maintenance (South East Asia, Saudi Electricity Company, KSA) EUSN, ENERGY & UTILITIES SECTOR NETWORK, Government of Canada, 2016 Connected+, IOT, Toronto, Canada September 2016 and 2015 Smart Grid, Smart Home HomeConnect, Toronto, Canada November 2014 Wind Power: a Cautionary Tale, Ontario Centre for Public Policy, 2010 POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Lubricants Blending and Quality Assurance (Accredited by the United Kingdom Lubricants Association (UKLA))
By EnergyEdge - Training for a Sustainable Energy Future
About this Virtual Instructor Led Training (VILT) Accredited by the United Kingdom Lubricants Association (UKLA), this 4 half-day Virtual Instructor Led Training (VILT) course will provide an in-depth understanding of the principles, economics and flexibility of lubricant blending plants and how to operate a lubricants blending plant efficiently and economically. The latest developments and trends in lubricant blending and the advantages and disadvantages of lubricant blending equipment, facilities and operations will be discussed. The importance of testing components and products for each blend, lubricant blend quality control and product quality management will also be explained. The VILT course will also clarify the importance of lubricant product filling, packaging and warehouse storage, strategies for optimising existing lubricant blending plant facilities and how to avoid or minimise problems with lubricant blending and product quality. The VILT course is recognised under the UKLA Continuing Professional Development (CPD) scheme for Registered Lubricant Professional. *There will be an examination for this VILT. Training Objectives This VILT course will enable you to: Learn about Mineral Oil Base Oils; API Groups I, II and III: Properties and Characteristics Acquire the knowledge about Synthetic Base Oils; API Groups IV and V: Properties and Characteristics Learn about Lubricant Additives: Properties and Characteristics Know the Lubricant Formulation and Ease of Blending Explore the Blending Plant Design: Grassroots Plants and Upgrading Existing Plants Learn about Blending Plant Equipment and Facilities and Their Operation Understand the Lubricant Blending Issues: Avoiding Problems Test and Analyse Base Oils and Additives Test and Analyse Blended Lubricants Explore the importance of Product Quality Control Understand the process of Lubricant Packaging and Filling Understand the process of Lubricant Storage Learn about Product Quality Management Target Audience This VILT course will be useful and applicable for: Middle and Senior managers to understand how and why to design and operate an efficient and profitable lubricant blending plant. Blending plant operators and specialists to improve and optimise current blending plant operations. Manufacturers of lubricants will understand how and why high quality components and effective testing during the entire blending process are important to final lubricant product quality and performance. Lubricant formulators will understand the importance of close communication and co-operation with blending plant managers and operators to minimise blending costs and to thereby maximise product profitability. Course Level Intermediate Training Methods The VILT course will be delivered online in 4 half-day sessions comprising 4 hours per day, with 2 breaks of 10 minutes per day. Course Duration: 4 half-day sessions, 4 hours per session (16 hours in total). Trainer Your expert course leader (CChem, MRC) has worked as Sales, Technical Marketing Manager and Company Director with over 50 years of broad experience in the lubricants, fuels, petroleum additives, with four leading companies Chevron, Ethyl Petroleum Additives Ltd, Texaco Limited and Kuwait Petroleum (GB) Ltd. His major recent responsibilities have been concerned with leading the Oil Industry Association United Kingdom Lubricants Association, and acting in an advisory capacity as Technical Director to the Association. He has acquired a wide experience in technical, marketing and sales within the oil industry. The related experience gained with the oil additives industry has provided him with special additional insights. He has also led the Certificate of Lubricant Competence course for the United Kingdom Lubricants Association (UKLA) for 11 years. He is a Chartered Chemist and a Member of the Royal Society of Chemistry. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information about post training coaching support and fees applicable for this. Accreditions And Affliations

Finance for Non-Finance Professionals in Oil & Gas Petroleum Fiscal Regimes & Applied Finance for Non-Finance Oil & Gas Professionals
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This separately bookable 3 full-day course is not designed to skill Oil & Gas engineers to be accountants, but to give the participants the confidence and ability to communicate with accountants and finance managers and to improve their own financial decision making. For technical professionals, a high level of single subject matter expertise is no longer sufficient for superior management performance. Oil & Gas technical professionals who wish to succeed in the resources industry are required to develop skills beyond their core functional knowledge. An understanding of financial information and management, and an awareness of the economic theory that drives value creation, are an integral part of the managers required suite of skills. This course can also be offered through Virtual Instructor Led Training (VILT) format. Training Objectives Workshop A: Finance for Non-Finance for Oil & Gas Professionals Attend this industry specific course and benefit from the following: Demystify financial jargon and fully interpret financial statements Understand Balance Sheets and Profit & Loss statements of Oil & Gas companies Discover the crucial distinction between cash flow and profit Understand how to make correct investment decisions using Net present Value and Internal Rate of Return Interpret oil and gas company financial reports using ratio analysis Learn the difference between cash costs and full costing of energy products Learn how to manage working capital for increased shareholder value Workshop B: Petroleum Fiscal Regimes and Applied Finance for Oil & Gas Industry Professionals Attend this advanced Training course to enhance your financial acumen from the following: Build and compare cash flow based models of both production sharing contract projects and royalty regime projects Gain an awareness of the different valuation methods for producing properties and undeveloped acreage Learn the industry specific accounting issues that apply when interpreting oil and gas company financial statements Understand how the physical characteristics of energy assets (e.g. reserves, reservoir quality) are translated into project valuations Learn how the investment analysts value oil and gas stocks and make buy/sell recommendations Target Audience This course is specifically designed for those with a non-finance background training from the Oil & Gas sector and requires only basic mathematical ability as a pre-requisite. It is presented in a manner that reduces the jargon to basic principles and applies them to numerous real-life examples. This course has been researched and developed for Managers, Superintendents, Supervisors, Engineers, Planners, Lawyers, Marketers, Team Leaders and Project Coordinators in the technical and non-technical departments in the Oil and Gas industry. Course Level Basic or Foundation Trainer Your expert course leader has presented over 300 courses and seminars in financial management. He began his career as a graduate in the Corporate Treasury of WMC Ltd having completed a degree in Applied Mathematics and Geology at Monash University. After five years with WMC, he pursued an MBA in finance and accounting at Cornell University in New York. He later gained a PhD in energy policy from the University of Melbourne. He worked for WMC Ltd in Perth as a Senior Financial Analyst in the Minerals Division and subsequently as an Energy Analyst in the Petroleum Division. In April 1997, he established an independent consultancy business providing advice to companies such as Woodside, Shell and Japan Australia LNG (MIMI). He spent many years as a consultant and commercial manager in the North West Shelf Gas project in Western Australia. Since 2006, he has been an Adjunct Fellow at the Macquarie University Applied Finance Centre where he teaches courses in valuation, financial statement modelling, and resources industry investment analysis. His background in geology and mathematics allows him to empathise with those who seek an understanding of finance but are approaching the learning experience with a technical mind. He receives consistently high ratings for his breadth of knowledge of the subject matter. He presents in a lively interactive style using real life examples and cases. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham