- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
2451 Courses in St Neots
Disciplined Agile Senior Scrum Master (DASSM): In-House Training
By IIL Europe Ltd
Disciplined Agile Senior Scrum Master (DASSM): In-House Training Do you want to take Disciplined Agile® to a new level? Are you looking for tools to solve complex problems and enhance your organization's agility? Do you want to learn how to lead your team to excellence? Expand your knowledge and build practical skills around Disciplined Agile®, business agility, leadership, and team development. Disciplined Agile Senior Scrum Master is a nine-lesson, instructor-led course that shows you how to use the Disciplined Agile tool kit to solve a variety of advanced problems, work with allies within your organization, and optimize how teams work. You will gain knowledge in planning, reporting and metrics, and coordinating activities, as well as how to meet challenges in these areas. And you'll develop the skills you need to foster emotional intelligence, resolve conflicts, and lead high-performance teams at any stage of development. Filled with activities, supplemental reading, and more, this course will prepare you to take the Disciplined Agile Senior Scrum Master (DASSM) exam and, equally important, start using Disciplined Agile immediately within your leadership role. What You Will Learn After the completion of this course, you will be able to: Accelerate your ability to lead high-profile initiatives that are critical to enterprise success Take a deep dive into the Disciplined Agile® tool kit to develop a comprehensive understanding of the hundreds of practices and strategies it contains and the trade-offs of applying them Apply the Disciplined Agile tool kit in hands-on exercises to guide your team in choosing and evolving your best way of working (WoW) in real-life situations Use the tool kit to solve complex challenges commonly encountered in development and operational teams, the value stream, and at the enterprise level Learn how to design and implement metrics that measure your improvements in areas where your teams are struggling Understand how to nurture emotional intelligence Feel confident empowering others on your team(s) Learn how to lead your teams in any situation to improve value delivery for your customers Apply the Disciplined Agile tool kit to guide your team in choosing and evolving the best way of working (WoW) in the situation you face Be prepared to take the Disciplined Agile® Senior Scrum Master (DASSM) exam and earn a valuable, credible certification Roles and responsibilities of DASSM Tuckman Team Development Model Emotional intelligence and why it is essential to team performance Business agility Tactical scaling factors in more complex situations Disciplined DevOps layer 'Test-first' method as it relates to the quality of requirements Scope and purpose of the value stream layer Coordinate activities process goal and why it is important Value creation structure of teams DA™ tool kit to optimize the flow of work and solve challenges related to coordinating and collaborating across teams, or within a larger team of teams Thomas-Kilmann Conflict Resolution Planning Five levels of scope Metrics

Certified Associate in Project Management (CAPM) Exam Prep: In-House Training
By IIL Europe Ltd
Certified Associate in Project Management (CAPM)® Exam Prep: In-House Training: In-House Training This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods Are you planning on taking the CAPM® examination? This course gives you the knowledge you need to pass the exam and covers CAPM®-critical information on project management theory, principles, techniques, and methods. You'll also have an opportunity for practical applications and time to review the kinds of questions you'll find in the CAPM® Exam. What you Will Learn Apply for the CAPM® Examination Develop a personal exam preparation plan Describe the structure, intent, and framework principles of the current edition of the PMBOK® Guide Explain the PMBOK® Guide Knowledge Areas, as well as their inter-relationships with the each other and the Process Groups Getting Started Program orientation The CAPM® certification process Certified Associate in Project Management (CAPM®) Examination Content Outline CAPM® eligibility requirements Code of Ethics and Professional Conduct Application options Foundation Concepts Skills and qualities of a project manager Project management terminology and definitions Relationship of project, program, portfolio, and operations management Project lifecycle approaches Project Integration Management Review Project Integration Management Knowledge Area Develop Project Charter Develop Project Management Plan Direct and Manage Project Work Manage Project Knowledge Monitoring and Controlling Perform Integrated Change Control Close Project or Phase Project Stakeholder Management Review Project Stakeholder Management Knowledge Area Identify Stakeholders Plan Stakeholder Engagement Manage Stakeholder Engagement Monitor Stakeholder Engagement Project Scope Management Review Project Scope Management Knowledge Area Plan Scope Management Collect Requirements Define Scope Create WBS Validate Scope Control Scope Project Schedule Management Review Project Schedule Management Knowledge Area Plan Schedule Management Define Activities Sequence Activities Estimate Activity Durations Develop Schedule Control Schedule Project Cost Management Review Project Cost Management Knowledge Area Plan Cost Management Estimate Costs Determine Budget Control Schedule Project Resource Management Review Project Resource Management Knowledge Area Plan Resource Management Estimate Activity Resources Acquire Resources Develop Team Manage Team Control Resources Project Quality Management Review Project Quality Management Knowledge Area Plan Quality Management Manage Quality Control Quality Project Risk Management Review Project Risk Management Knowledge Area Plan Risk Management Identify Risks Perform Qualitative Risk Analysis Perform Quantitative Risk Analysis Plan Risk Responses Implement Risk Responses Monitor Risks Project Communications Management Review Project Communications Management Knowledge Area Plan Communications Management Manage Communications Monitor Communications Project Procurement Management Review Project Procurement Management Knowledge Area Plan Procurement Management Conduct Procurements Control Procurements Summary and Next Steps Program Review Mock CAPM® Exam Getting Prepared for the CAPM® Exam After the CAPM® Exam

Certified ScrumMaster: In-House Training
By IIL Europe Ltd
Certified ScrumMaster®: In-House Training This course covers Scrum and the principles and tools required to be an effective ScrumMaster. You will come away with a good understanding of the Scrum framework and the underlying principles required to make effective decisions regarding the application of Scrum to different situations. At the end of the course, you will receive membership to the Scrum Alliance for two years and, following completion of an online test, will become a Scrum Alliance Certified ScrumMaster®. Our Certified Scrum Trainers pay the initial, two-year membership fee for each student who successfully completes our Certified ScrumMaster® course. This membership fee also covers the cost of the CSM Test. A link to the test will be sent to you following your course. The CSM test has a passing score of 37 out of 50 questions within a 60-minute timeframe. You will have two attempts within 90 days after you receive your welcome e-mail to pass the test at no cost. After two attempts or 90 days, you will be charged $25 for each additional attempt. What you will Learn At the end of this program, you will be able to: Provide a clear understanding of the fundamental principles of Scrum Use the principles, practices, and tools required to be an effective ScrumMaster Make effective decisions regarding the application of the Scrum framework to different situations, including: Practical, project-proven practices The essentials for getting a project off on the right foot How to write user stories and structure your product backlog How to help both new and experienced teams be more successful How to successfully scale Scrum Tips and tricks from the instructor's many years of using Scrum in a wide variety of environments Getting Started Introduction Course structure Course goals and objectives Agile Principles and Scrum Overview Agile Principles Lean Principles Process control models Incremental and Iterative development Shifting the focus on product management Overview of the Scrum process The Team Dedicated cross-functional teams T-shaped people Sprint Planning Team capacity Facilitating the Sprint Planning meeting The Sprint backlog Sprint Burndown chart Scrum Roles and Responsibilities The team and building effective teams ScrumMaster responsibilities Product Owner responsibilities The Scrum project community What happens to traditional roles in Scrum? Scrum Meetings Daily Scrum Reviews Retrospectives Product Backlog and User Stories Product backlog characteristics User stories Getting your first backlog Getting backlog items ready Slicing User stories Estimation for Forward Planning Why comparative estimation works Planning poker Affinity estimation Release Planning and Tracking Progress Velocity Release planning Tracking release progress Scaling Scrum Scrum of Scrums Scaling the product backlog Scaling across a program and business areas Distributed teams

Quality Assurance for Good Laboratory Practice
By Research Quality Association
Course Information A must-have programme for Quality Assurance auditors stepping into or honing their role within a Good Laboratory Practice (GLP) environment, this course offers invaluable, expert guidance for crafting a robust and efficient GLP audit programme. What will I learn? A solid regulatory foundation underpinning quality assurance activities Clarity on the roles of Quality Assurance, management, and study director within the framework of Good Laboratory Practice principles Enhanced efficacy in inspections and audits Heightened compliance with Good Laboratory Practice standards for your facility Unique insights into governmental monitoring activities within the GLP sphere. This course is structured to encourage delegates to Discuss and develop ideas Solve specific problems Examine particular aspects of GLP. Tutors Tutors will be comprised of (click the photos for biographies): Cate Ovington Director, The Knowlogy Group Ltd Jane Elliston Senior Quality Assurance Auditor, Battelle UK Shona Ross Head of QA, Tower Mains Ltd Programme Please note timings may be subject to alteration. Day 1 09:00 Welcome and Introductions 09:15 Good Laboratory Practice Standards and Regulations An insight into the background and history of Good Laboratory Practice. 09:45 Principles of Quality Assurance What is the role and responsibilities of QA in GLP. Maintaining the independence of QA and what is an audit. 10:30 Break 10:45 Standard Operating Procedures GLP requirements and QA involvement. 11:30 Study Plans GLP requirements and QA involvement. 12:05 QA Programme Risk based programme, what are study, process and facility audits. 13:00 Lunch 14:00 Inspections Attitudes, techniques and attributes. 14:40 Workshop 1 - Facility and Process Inspections An exercise in inspection planning and preparation for inspections. 15:15 Break 15:30 Workshop 1 - Feedback 15:45 The Auditor and Audit Conduct Attitudes, attributes and techniques. 16:30 Panel Session An opportunity for delegates to put questions to the panel of speakers. 17:15 Close of Day Day 2 09:00 Workshop 2 - A Mock Audit 10:45 Break 11:00 Workshop 2 - Feedback 11:30 Auditing the Study Report Techniques and methods for the QA audit of the study report. 12:00 Record Keeping and Data The impact of GLP on data and records management. 12:40 Lunch 13:25 Data Integrity A look at the OECD GLP guidance document; the expectations of the regulators and the involvement of QA - Where QA adds value. 14:15 Workshop 3 - Amendments to Study Plan and Deviations from the Plan What are they? What is the difference between them? How are they controlled? 15:00 Workshop 3 - Feedback 15:15 Break 15:30 Regulatory Compliance GLP Monitoring Authority monitoring for compliance with Good Laboratory Practice. 16:15 Panel Session An opportunity for delegates to put questions to the panel of speakers. 16:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Learn

This Level 4 City and Guilds 2396-01 Design and Verification of Electrical Installations course has been designed to help develop the skills and up date the knowledge of the requirements to enable you to professionally design, erect and then verify an electrical installation. This course is aimed at those who will have responsibility for designing, supervising, installing and testing electrical installations. Further information can be found here: C&G 2396 Electrical Design Course — Optima Electrical Training (optima-ect.com)

Good Laboratory Practice for Study Directors, Principal Investigators, Study Staff and Management
By Research Quality Association
Course Information Embark on our GLP course offering extensive guidance and pragmatic support tailored for individuals serving as Study Directors or Principal Investigators overseeing non-clinical safety studies on pharmaceuticals, agricultural, and industrial chemicals within the realm of Good Laboratory Practice (GLP). This comprehensive programme extends its benefits to study staff and management operating in GLP-compliant environments. The course extensively covers the current OECD GLP Principles and UK GLP legislation, while also referencing international standards, regulations, and guidelines pertinent to the field. Benefits of this course: Practical help and guidance on the interpretation and application of GLP An opportunity to update your knowledge of GLP with the current interpretation of requirements Access to an experienced panel of speakers Information on how other organisations address GLP issues An opportunity to improve your understanding of the GLP requirements as they are applied in different situations. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of GLP Learn from the experience of others. Tutors Tutors will be comprised of (click the photos for biographies): Tim Stiles Consultant, Qualogy Ltd Tony Woodall Head of Quality Assurance, Alderley Analytical Gill Armour Study Monitor Team Leader, AstraZeneca Jane Elliston Senior Quality Assurance Auditor, Battelle UK Vanessa Grant -, - Jeanet Logsted CEO, Scantox Programme Please note timings may be subject to alteration. Day 1 09:00 Registration 09:15 Welcome and Introductions 09:35 Development of Good Laboratory Practice A review of the history of GLP, its current scope and application, with a synopsis of current European and international standards. 10:05 Roles and Responsibilities The responsibilities of study director, test facility, management and study staff in the conduct of a GLP study. 10:45 Break 11:00 The Roles and Responsibilities of the Study Director and Test Facility Management The role of the study director in the management and control of a study, as defined by GLP, and management's roles are explored. 11:45 Multi-site Studies What is a multi-site study and when should such concepts be applied on a study. The role of the study director and principal investigator in the planning, conduct and reporting of multi-site study are explored. 12:30 Study Plan (Protocols) GLP requirements for the preparation of a study plan, content, authorisation, amendments and deviations are discussed. 13:00 Lunch 13:45 Workshop 1 - The Study Plan Some practical problems with study plans and amendments explored. 14:45 Workshop 1 - Feedback 15:00 Standard Operating Procedures The control, content and authorisation of SOPs and the principles behind the practice. 15:30 Break 15:45 Workshop 2 - Practical Study Conduct Problems Dealing with practical problems encountered during the conduct of studies. 16:40 Workshop 2 - Feedback 17:15 Close of Day Day 2 09:00 Questions and Answers Discussion of issues raised by course delegates. 09:20 Quality Assurance The interactions between QA, management, study director and principal Investigator are discussed as is QAs role when conducting a multi-site study. 10:00 The Final Report The content of the final report and the role of those involved in its preparation and approval. Specific reporting requirements when conducting a multi-site study are also explained. 10:30 Break 10:45 Workshop 3 - Final Report Problems Practical problems of report preparation including compliance statements. 11:30 Workshop 3 - Feedback 12:00 Management of Raw Data and Records A view on how records and materials are managed and archived in compliance with GLP. 12:45 Lunch 13:30 Workshop 4 - Data and Sample Management Issues Dealing with data and sample management issues. 14:15 Workshop 4 - Feedback 14:45 Regulatory Inspection Government monitoring for compliance with Good Laboratory Practice. 15:15 Panel Session This panel session will address any outstanding issues raised by delegates. 15:45 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

High Impact Media Communication Programme
By Mpi Learning - Professional Learning And Development Provider
Develop confidence & capability in delivering to the camera & conducting interviews. Develop your own personal brand & impact on camera whether in person 'live', pre-recorded or online.

The Magic of Mentoring
By Mpi Learning - Professional Learning And Development Provider
In this course you will understand what mentoring is about, understand your roles in the process. Gain self-insight into your own interpersonal style and way of relating to others that may affect mentoring interactions.

Gold CSCS Card NVQ Level 3 Diploma in Construction Contracting Operations This qualification is designed for those in a technical job role, This qualification is appropriate for employees in the construction and built environment sector working at a technical level in disciplines such as estimating, buying, planning, surveying, site technical support and design co-ordination. You could be site-based and have responsibility for functions such as dimensional control, surveys, physical testing, and work planning. You may work across one or more of the following pathways: Estimating Buying Planning Surveying Site Technical Support Design Co-Ordinator General Induction - As soon as you register you will be given a dedicated assessor. They will arrange an induction and together with your assessor, you will get to decide on the pathway which best proves your competency. The induction is used to plan out how you will gather the relevant evidence to complete the course. During the course - The assessor will work with you to build a portfolio of evidence that allows you to showcase your knowledge, skills and experience. The assessor will also regularly review and provide you with feedback. This will allow you to keep on track to progress quickly. You will be assessed through various methods such as observations, written questions, evidence generated from the workplace, professional discussion and witness testimonials. On completion - Once all feedback has been agreed, the Internal Quality Assurer will review your portfolio and in agreement with your assessor the certificate will be applied for. To download our PDF for this course then please click here.

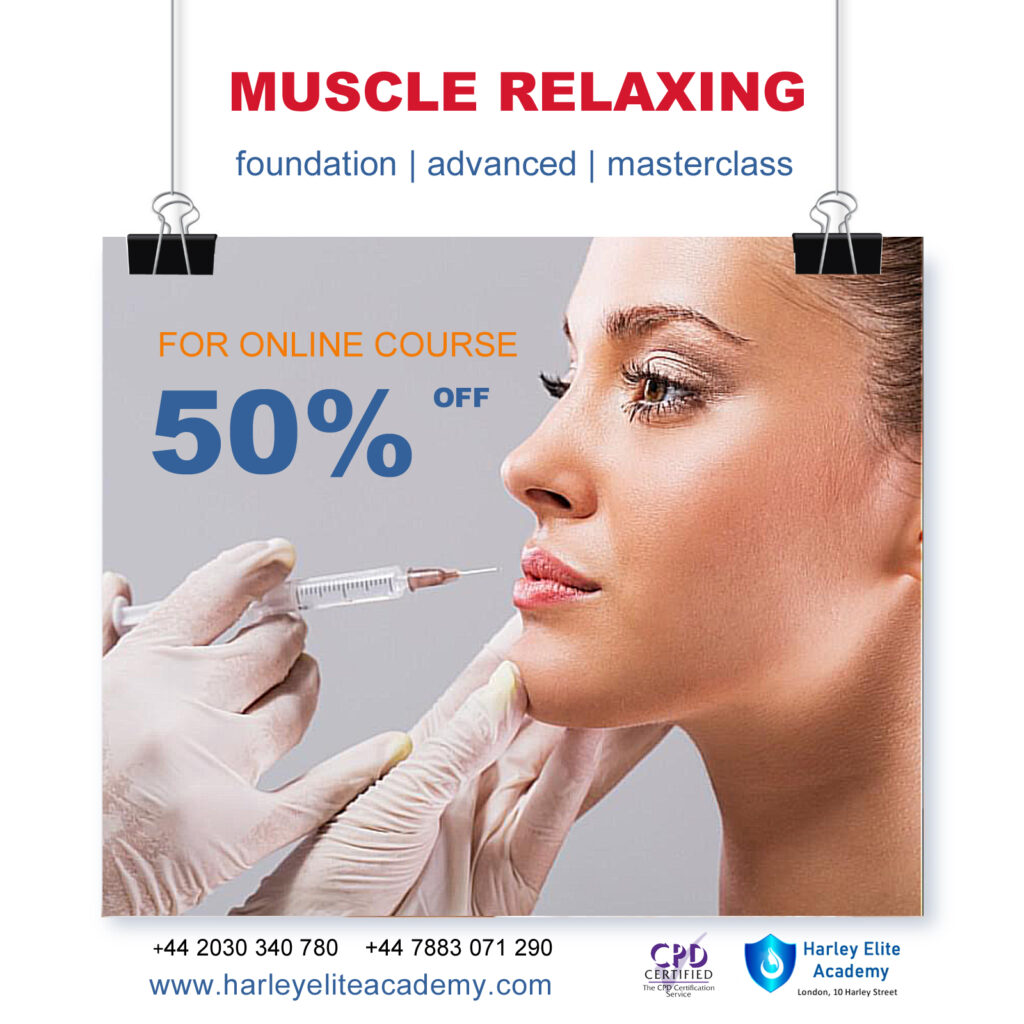
MUSCLE RELAXING | BOTOX®
By Harley Elite Academy (HeLa)
Foundation • Advanced • Masterclass 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) COURSE LEVEL BEGINNER | Foundation Course, INTERMEDIATE | Advanced Course, EXPERT | Masterclass Course
Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham