- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1941 Courses in Nottingham
The Emergency treatment of Adrenal Crisis
By Guardian Angels Training
Gain essential knowledge and skills to recognise, manage, and provide prompt emergency treatment for adrenal crisis with our course. Ideal for healthcare professionals.

An Understanding of Seizure First Aid and the Administration of Rectal Paraldehyde
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in seizure first aid and rectal paraldehyde administration with our course. Ideal for caregivers, healthcare professionals, educators, and individuals.

Leading SAFe: In-House Training
By IIL Europe Ltd
Leading SAFe®: In-House Training During this course, attendees gain the knowledge necessary to lead a Lean-Agile enterprise by using the Scaled Agile Framework® (SAFe®) and its underlying principles derived from Lean, systems thinking, Agile development, product development flow, and DevOps. Participants in the class gain insights into mastering business agility to thrive in competitive markets. They discuss how to establish team and technical agility and organize and re-organize around the flow of value. Attendees also learn and practice the skills to support and execute PI Planning events and coordinate multiple Agile Release Trains (ARTs). Class participants will explore the importance of adopting a customer-centric mindset and Design-Thinking approach to Agile Product Delivery. Learners will also understand how to implement a Lean Portfolio Management function within their enterprise. What you will Learn After attending this class, attendees should be able to: Lead the transformation to business agility with SAFe® Become a Lean-Agile leader Understand customer needs Design Thinking Enable Agile Product delivery Implement Lean Portfolio Management Thrive in the digital age with business agility Become a Lean-Agile leader Establish Team and Technical Agility Build solutions with Agile Product Delivery Explore Lean Portfolio Management Lead the change Become a Certified SAFe® Agilist

For Estate Agents - Identify the problem to ascertain the remedy.
By Mark Bentley PPNAEA (Mark Bentley Ltd)
This course will help you find remedies to a lack of new business or a lack of property sales etc etc

Certified Scrum Product Owner: In-House Training
By IIL Europe Ltd
Certified ScrumMaster®: In-House Training This course is an introduction to Scrum and the principles and tools required to be an effective Scrum Product Owner. You will come away with a good understanding of the Scrum framework and the underlying principles required to make effective decisions regarding the application of the Scrum framework to different situations. Participants successfully completing this course earn a Certified Scrum Product Owner® (CSPO®) designation. The Scrum Alliance certification includes a one-year membership with Scrum Alliance. What You Will Learn You'll learn how to: Use the principles, practices, and tools required to be an effective Scrum Product Owner Make effective decisions regarding the application of the Scrum framework to different situations, including: Setting product vision and goals Chartering the project Writing user stories and structuring your product backlog Scaling the Product Owner Estimating for forward planning Applying prioritization techniques Planning and tracking release progress Getting Started Introduction Course structure Course goals and objectives Agile Principles and Scrum Overview Process control models Incremental and iterative development Shifting the focus on product management Overview of the Scrum process Agile principles Lean principles Scrum Roles and Responsibilities Scrum roles Cross-functional teams Product Owner Responsibilities The Scrum Project Community What happens to my traditional role in Scrum? Chartering the Project Establishing a shared vision Elevator Statement Data sheets Product Vision Box Magazine Review / Press Release Product Backlog and User Stories Product uncertainty and progressive refinement User role modeling User Stories Product backlog characteristics Getting backlog items ready Slicing User Stories Using the product backlog to manage expectations Sprints Done and Scaling Done The Scrum process in detail Sustainable pace The Product Owner's role in each of the Scrum meetings Scaling the Product Owner Scaling Scrum Approaches to scaling the Product Owner Estimation for Forward Planning Why comparative estimation works Planning Poker Affinity Estimation Prioritization Techniques Additional Product Backlog Prioritization Techniques Kano Analysis Theme Screening Release Planning and Tracking Progress Velocity Release Planning Tracking release progress

SAFe Product Owner / Product Manager: In-House Training
By IIL Europe Ltd
SAFe® Product Owner / Product Manager: In-House Training Develop the skillsets needed to guide the delivery of value in a Lean Enterprise by becoming a SAFe® 5.0 Product Owner / Product Manager (POPM). During this course, attendees gain an in-depth understanding of how to effectively perform their role in the Agile Release Train (ART) as it delivers value through Program Increments. Attendees explore how to apply Lean thinking to decompose Epics into Features and Stories, refine Features and Stories, manage Program and Team backlogs, and plan and execute Iterations and Program Increments. Attendees also discover how the Continuous Delivery Pipeline and DevOps culture contribute to the relentless improvement of the ART. What you will Learn To perform the role of a SAFe® Product Owner / Product Manager, attendees should be able to: Articulate the Product Owner and Product Manager roles Connect SAFe® Lean-Agile principles and values to the PO / PM roles Decompose Epics into Features and decompose Features into Stories Manage Program and Team backlogs Collaborate with Agile teams in estimating and forecasting work Represent customer needs in Program Increment Planning Execute the Program Increment and deliver continuous value Becoming a Product Owner / Product Manager in the SAFe® enterprise Preparing for PI Planning Leading PI Planning Executing Iterations Executing the Program Increment Becoming a Certified SAFe® Product Owner / Product Manager

Carrying out successful property valuations/marketing appraisals
By Mark Bentley PPNAEA (Mark Bentley Ltd)
By the end of the course you will have picked up numerous tips and guidance and advice to help you carry out great property valuations/market appraisal's which will help you improve you conversion rate and win more clients.

Microsoft Excel Training
By FourSquare Innovations Ltd
FourSquare Training specialise in private, corporate Microsoft Excel courses delivered at your premises and tailored to your needs.

An Understanding of fine-bore Nasogastric Tube Insertion
By Guardian Angels Training
Gain comprehensive knowledge and practical skills for safe and effective fine-bore nasogastric tube insertion with our healthcare professional course.

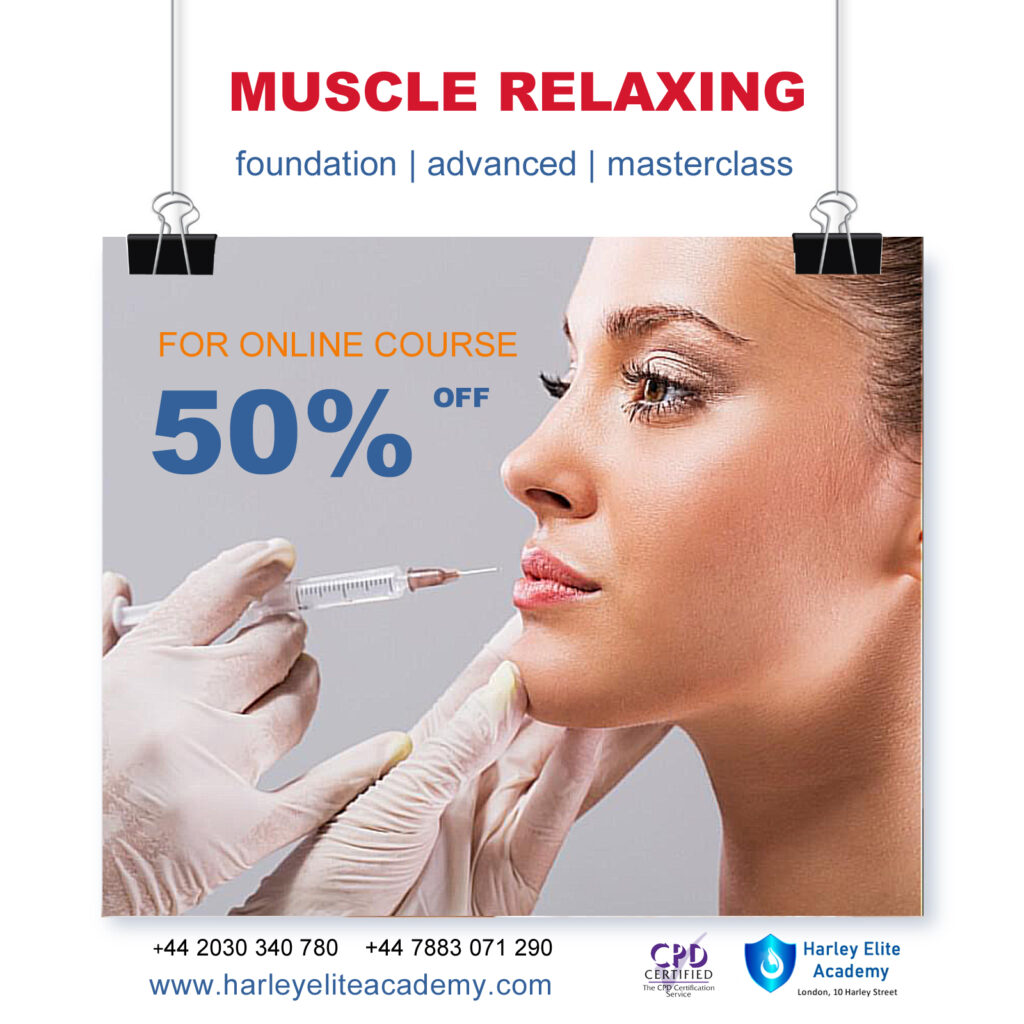
MUSCLE RELAXING | BOTOX®
By Harley Elite Academy (HeLa)
Foundation • Advanced • Masterclass 8 CPD POINTS 1 DAY INTENSIVE COURSE ONLINE or IN-CLINIC NOTE! After booking we will contact you for scheduling the exact course date! Courses dates are subject to change due to mentors availability. We will inform you via email if a date becomes available! You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. Additional information ATTENDANCE ONLINE (theory), IN-CLINIC (Practice) COURSE LEVEL BEGINNER | Foundation Course, INTERMEDIATE | Advanced Course, EXPERT | Masterclass Course
Search By Location
- course Courses in London
- course Courses in Birmingham
- course Courses in Glasgow
- course Courses in Liverpool
- course Courses in Bristol
- course Courses in Manchester
- course Courses in Sheffield
- course Courses in Leeds
- course Courses in Edinburgh
- course Courses in Leicester
- course Courses in Coventry
- course Courses in Bradford
- course Courses in Cardiff
- course Courses in Belfast
- course Courses in Nottingham