- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3344 Healthcare courses delivered Online
Enhance your professional skills in Health & Care with our Mandatory Refresher Training. Covering critical areas from First Aid to Mental Health, this training is designed to update and deepen your knowledge in Health Care settings. Ensure compliance and excellence in Care with our comprehensive course offerings. Learning Outcomes Administer effective First Aid as a Health Care professional. Apply specialised paediatric First Aid techniques in Care settings. Understand comprehensive Care Certificate standards for Health & Care. Advance in Health & Care with a Level 3 Diploma in Health & Social Care. Fulfill Duty of Care obligations with updated practices. Enhance communication within Health Care settings. Manage information securely and efficiently in Care environments. Implement robust infection control protocols in Health & Care. Promote equality and inclusion within Care settings. Adopt person-centred approaches to improve Care quality. Address mental health issues with confidence in Health Care. This Mandatory Refresher Training Bundle comes up with the following courses:Course 01: First Aid at Work Essentials of First Aid in Health & Care: Equip Health Care professionals with the skills necessary to respond to emergencies effectively. Course 02: Paediatric First Aid Specialised First Aid for Children: Focus on paediatric emergencies and preventative measures in Care settings. Course 03: Care Certificate Foundations of Professional Care: Standardized training fulfilling the Care Certificate requirements to ensure high-quality Health & Care services. Course 04: Level 3 Diploma in Health & Social Care Advanced Practices in Health & Care: Enhance your qualifications with advanced knowledge crucial for Health & Care professionals. Course 05: Duty of Care Ethical and Legal Practices in Care: Understand and apply Duty of Care to safeguard and promote the interests of those receiving Care. Course 06: Communication in Care Settings Effective Communication Techniques: Master communication skills to improve interactions and understanding within Health Care environments. Course 07: Information Handling in Care Settings Confidentiality and Data Management: Training on secure handling of information in compliance with Care standards. Course 08: Infection Prevention and Control in Care Settings Maintaining Safe Care Environments: Strategies to prevent and control infections, critical for patient and staff safety in Health Care settings. Course 09: Equality and Inclusion in Care Settings Promoting Diverse and Inclusive Care: Address and foster equality and inclusion practices within Health Care facilities. Course 10: Person Centred Approaches in Care Settings Individualized Care Strategies: Emphasize the importance of person-centred Care to enhance patient satisfaction and outcomes. Course 11: Mental Health Mental Health Awareness and Support: Equip staff with the skills to recognize and address mental health issues effectively in Health & Care settings.
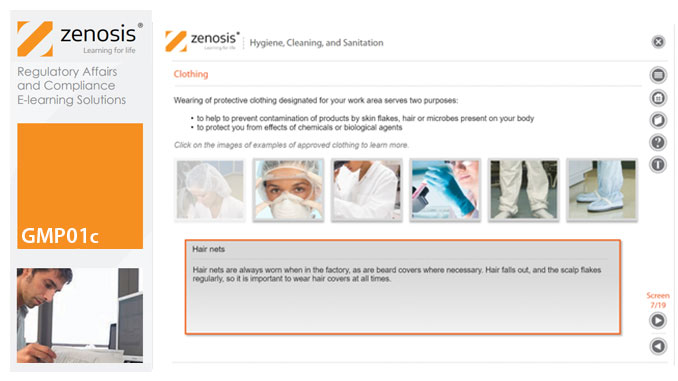
GMP01c - Hygiene, cleaning, and sanitation
By Zenosis
Prevention of contamination is one of the most important goals of GMP. Contamination of product is often difficult to detect, so GMP rules emphasise preventive measures, including: attention to personal health and hygiene, and the wearing of special clothing, by staff; and cleaning and sanitation of premises and equipment. In this short course we set out the basics of GMP requirements in these vital areas.
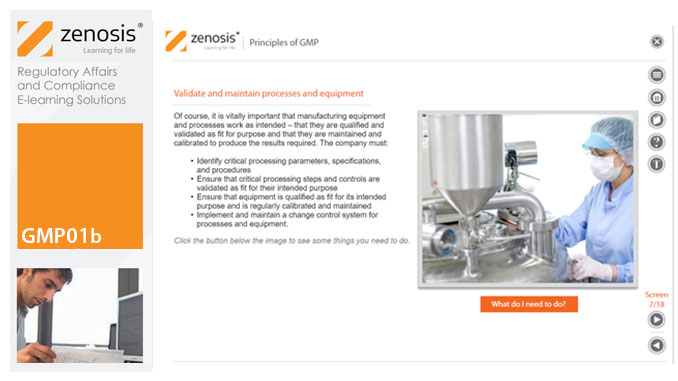
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
GMP01a - GMP – what and why
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. Everyone who works in a processing, quality control, packaging, or warehouse environment for a pharmaceutical or biotechnology company, or one of their contractors, must understand why GMP is important, how it applies to them, and how to comply with it. This short course explains what GMP is and why it is important, and it gives some lessons from history. It introduces the regulations and guidance documents that are the source of GMP rules. Finally, it touches on regulatory inspections and the consequences that can arise from failure to comply with GMP requirements.

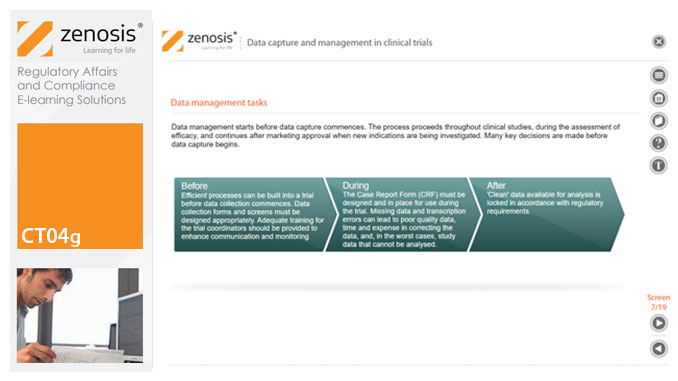
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
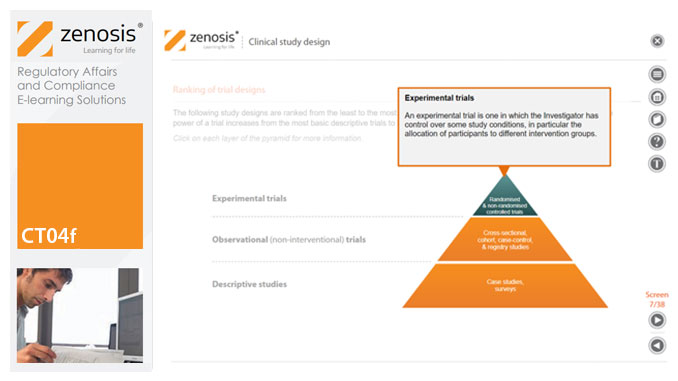
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
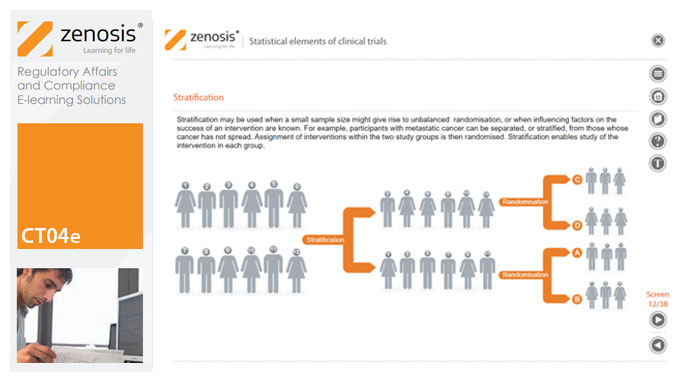
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
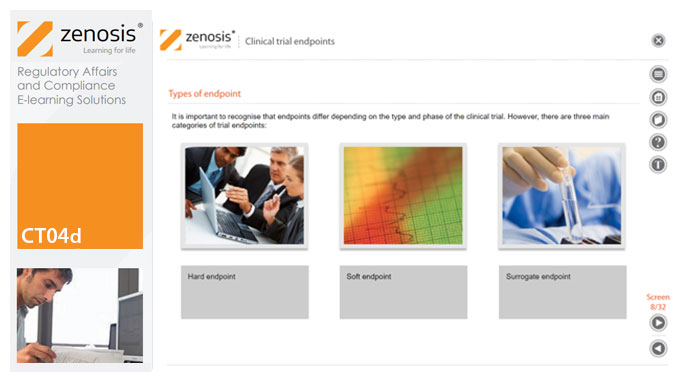
CT04d - Clinical trial endpoints
By Zenosis
In clinical trials, endpoints are measurements to evaluate the results of a new treatment, at an individual patient level. The study data can be extrapolated to patient populations on the basis of clinical similarities to patients participating in the trial. When clinical trial data have been obtained, focus is on the trial endpoints; more specifically, the focus is on whether the trial met or failed the primary endpoint specified before the trial started. The purpose and various types of endpoints are discussed in this short course.
CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
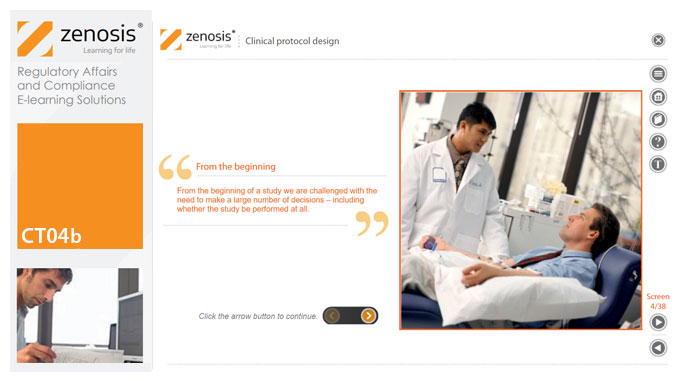
CT04b - Clinical protocol design
By Zenosis
Clinical trial protocols are an essential part of clinical trial design. Protocol documents are critical to conducting safe and cost-effective investigations. Protocol documents are large and complex, containing comprehensive information relating to purpose, design and conduct of a clinical trial. Aspects of a protocol include patient eligibility criteria, and treatment specifications. This short course provides an overview of clinical trial protocols. Opportunities to improve a clinical trial protocol for regulatory approval are also discussed.