- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3437 Healthcare courses in Cardiff delivered On Demand
SAM03: Consumer-directed Advertising and Online Promotion of Prescription Drugs in the USA
By Zenosis
Unlike the great majority of other countries, the advertising of prescription drugs directly to consumers is permitted in the USA. Spending by drug companies on direct-to-consumer (DTC) advertising of prescription drugs has increased more than four-fold over two decades, with a dramatic increase in the number of TV ads. In addition, the Internet and social media platforms have increasingly enabled companies to engage more actively with the public.

Supporting Children and Young People with Disabilities - 8 Courses Bundle
By NextGen Learning
Are you ready to embark on an enlightening journey of wisdom with the Supporting Children and Young People with Disabilities bundle, and pave your way to an enriched personal and professional future? If so, then Step into a world of knowledge with our bundle - Supporting Children and Young People with Disabilities. Delve into eight immersive CPD Accredited courses, each a simple course: Course 01: Disability Assessor Training Course 02: Autism Diploma Course 03: ADHD Course 04: Dyslexia Course 05: SEN Teaching Assistant Course 06: EYFS Teaching Diploma Course 07: Advanced Safeguarding Children (Level 2 Safeguarding) Course 08: Family Support Worker Traverse the vast landscapes of theory, unlocking new dimensions of understanding at every turn. Let the Supporting Children and Young People with Disabilities bundle illuminate your path to wisdom. The Supporting Children and Young People with Disabilities bundle offers a comprehensive exploration into a rich tapestry of vast knowledge across eight carefully curated courses. The journey is designed to enhance your understanding and critical thinking skills. Each course within the bundle provides a deep-dive into complex theories, principles, and frameworks, allowing you to delve into the nuances of the subject matter at your own pace. In the framework of the Supporting Children and Young People with Disabilities package, you are bestowed with complimentary PDF certificates for all the courses included in this bundle, all without any additional charge. Adorn yourself with the Supporting Children and Young People with Disabilities bundle, empowering you to traverse your career trajectory or personal growth journey with self-assurance. Register today and ignite the spark of your professional advancement! So, don't wait further and join the Supporting Children and Young People with Disabilities community today and let your voyage of discovery begin! Learning Outcomes: Upon completion of the Supporting Children and Young People with Disabilities Bundle, you will be able to: Attain a holistic understanding in the designated areas of study with the Supporting Children and Young People with Disabilities bundle. Establish robust bases across each course nestled within the Supporting Children and Young People with Disabilities bundle. Decipher intricate concepts through the articulate content of the Supporting Children and Young People with Disabilities bundle. Amplify your prowess in interpreting, scrutinising, and implementing theories. Procure the capacity to engage with the course material on an intellectual and profound level. Become proficient in the art of problem-solving across various disciplines. Stepping into the Supporting Children and Young People with Disabilities bundle is akin to entering a world overflowing with deep theoretical wisdom. Each course within this distinctive bundle is an individual journey, meticulously crafted to untangle the complex web of theories, principles, and frameworks. Learners are inspired to explore, question, and absorb, thus enhancing their understanding and honing their critical thinking skills. Each course invites a personal and profoundly enlightening interaction with knowledge. The Supporting Children and Young People with Disabilities bundle shines in its capacity to cater to a wide range of learning needs and lifestyles. It gives learners the freedom to learn at their own pace, forging a unique path of discovery. More than just an educational journey, the Supporting Children and Young People with Disabilities bundle fosters personal growth, enabling learners to skillfully navigate the complexities of the world. The Supporting Children and Young People with Disabilities bundle also illuminates the route to a rewarding career. The theoretical insight acquired through this bundle forms a strong foundation for various career opportunities, from academia and research to consultancy and programme management. The profound understanding fostered by the Supporting Children and Young People with Disabilities bundle allows learners to make meaningful contributions to their chosen fields. Embark on the Supporting Children and Young People with Disabilities journey and let knowledge guide you towards a brighter future. CPD 80 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Individuals keen on deepening their firm understanding in the respective fields. Students pursuing higher education looking for comprehensive theory modules. Professionals seeking to refresh or enhance their knowledge. Anyone with a thirst for knowledge and a passion for continuous learning. Requirements Without any formal requirements, you can delightfully enrol in this Supporting Children and Young People with Disabilities Bundle. Career path Armed with the Supporting Children and Young People with Disabilities bundle, your professional journey can reach new heights. The comprehensive theoretical knowledge from this bundle can unlock diverse career opportunities across several fields. Whether it's academic research, consultancy, or programme management, this bundle lays a solid groundwork. Certificates CPD Certificate Of Completion Digital certificate - Included 8 Digital Certificates Are Included With This Bundle CPD Quality Standard Hardcopy Certificate (FREE UK Delivery) Hard copy certificate - £9.99 Hardcopy Transcript: £9.99

Level 3 Diploma in Store Assistant - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

Enhance your professional toolkit with our comprehensive 'Restraint Awareness: Safeguarding Yourself and Others' course. Acquire crucial skills in risk assessment, communication, and post-incident management. Ideal for healthcare, security, and education professionals seeking ethical and effective strategies for conflict resolution. Elevate your expertise and prioritize safety with our expert-led training. Enroll now for a transformative learning experience.

Explore the intricacies of Clinical Research Administration with our comprehensive course on Protocols and Ethics. Gain expertise in trial design, ethical considerations, regulatory compliance, and more. Prepare for a future in clinical research with practical insights and knowledge. Enroll now for a rewarding journey in advancing healthcare through responsible and ethical clinical trials.

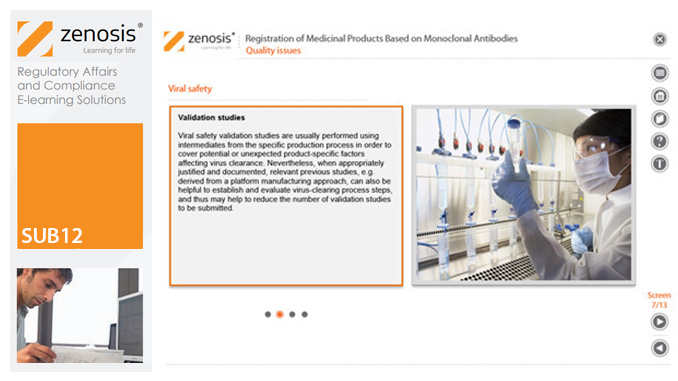
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
PV04: Signal Detection and Management in Pharmacovigilance
By Zenosis
This module provides a guide to signal detection and management for approved products. The subject is presented as a process comprising four stages: signal detection, signal validation, signal analysis and prioritisation, and risk assessment and minimisation.

SUB04: Preparing Submissions in the Common Technical Document (CTD) Format
By Zenosis
The CTD is the internationally recognised standard format for submissions to medicines regulatory authorities. In the European Economic Area, the USA and Canada, the CTD, in its electronic format (eCTD), is mandatory for all applications for marketing approval and all subsequent related submissions. The CTD is accepted in many other countries, being mandatory for new prescription medicines in some. This module explains the rationale for the CTD and provides guidance on its structure and format and the ways in which it is used.

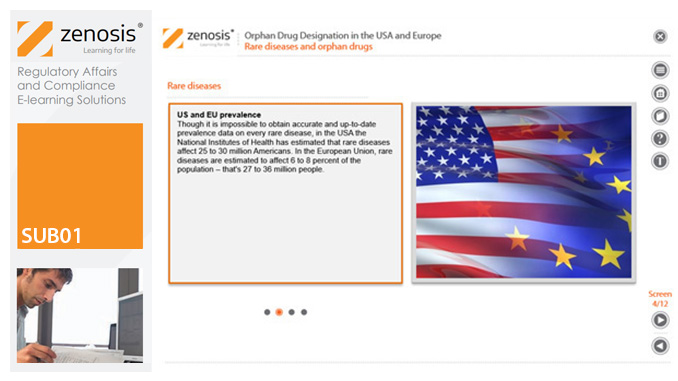
SUB01: Orphan Drug Designation in the USA and Europe
By Zenosis
Medicines for the prevention, diagnosis, or treatment of rare diseases have become known as ‘orphan drugs’ because of their commercial unattractiveness. Development of such products is successfully encouraged through incentives offered by regulatory authorities. To qualify for important incentives, the sponsor of a drug must gain ‘orphan designation’ for its use in an indication. This module describes the requirements for orphan designation and how to apply for it in the USA and the European Economic Area.
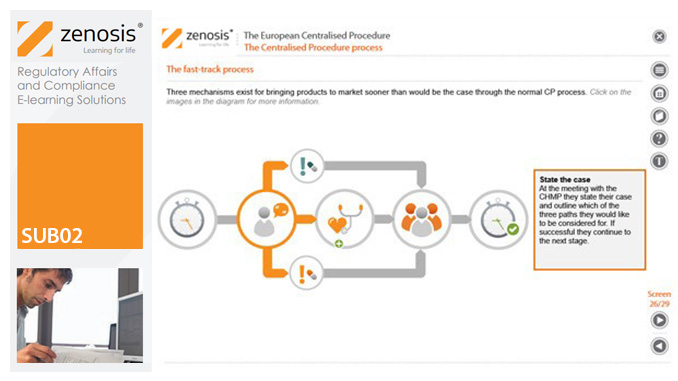
SUB02: The European Centralised Procedure (CP)
By Zenosis
The Centralised Procedure (CP) is one of three routes available to applicants to gain multinational marketing authorisation within the European Economic Area (EEA) on the basis of a single application. In the CP, one successful application leads to a marketing authorisation being issued by the European Commission that applies throughout the EEA. The CP is mandatory for certain types of products.