- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
130 Good Manufacturing Practice (GMP) courses
Welcome to the Product Development and Supply Chain Management Course Bundle, a comprehensive program that combines a diverse range of courses to equip you with the skills and knowledge needed to excel in the dynamic fields of product development and supply chain management. In this bundle, you will gain expertise in areas such as good manufacturing practice, digital product management, production management, quality assurance, sales and marketing, warehouse management, and more. Data shows that the demand for skilled professionals in product development and supply chain management is on the rise, with job opportunities projected to increase by 15% over the next five years. By enrolling in this course bundle, you will learn how to navigate the complexities of product development and supply chain management, gain deep insights, and acquire industry-relevant skills that are highly sought after by employers. Besides, learn to optimise production processes, ensure quality control, develop effective marketing strategies, manage warehouses safely, and streamline supply and procurement operations. With real-world case studies, examples, and interactive learning, you will be equipped with the tools and knowledge needed to excel in the competitive world of product development and supply chain management. Join us now and take your career to new heights with the Product Development and Supply Chain Management Course Bundle! This Product Development and Supply Chain Management Bundle Package includes: Course 01: Diploma in Product Development at QLS Level 4 10 Premium Additional CPD QS Accredited Courses - Course 01: Good Manufacturing Practice Course 02: Digital Product Management Training Course 03: Production Manager Course 04: Level 3 Diploma in Supply Chain Management Course 05: Quality Assurance (QA) Management Course 06: Sales and Marketing Diploma Course 07: Warehouse Management Course 08: Warehouse Safety Training Course 09: Advanced Training on Supply and Procurement Course 10: Material Management Why Prefer This Product Development and Supply Chain Management Bundle? You will receive a completely free certificate from the Quality Licence Scheme Option to purchase 10 additional certificates accredited by CPD Get a free Student ID Card - (£10 postal charges will be applicable for international delivery) Free assessments and immediate success results 24/7 Tutor Support After taking this Product Development and Supply Chain Management bundle courses, you will be able to learn: Understand and apply good manufacturing practice principles to ensure quality and compliance in production processes. Develop effective digital product management strategies to drive innovation and market success. Manage production processes and resources efficiently as a production manager. Implement quality assurance (QA) management practices to ensure product quality and safety. Create and implement sales and marketing strategies to drive business growth. Efficiently manage warehouses and ensure safety compliance. Gain advanced knowledge of supply chain management and procurement processes for optimal operations. Implement effective material management strategies to optimise inventory and supply chain performance. ***Curriculum breakdown of Product Development*** Module 01: Introduction to Product Management Module 02: Product Classification Module 03: Developing the Product Plan Module 04: New Product Development Module 05: Levels of a Product and Product Life Cycle Module 06: Product Pricing Strategy Module 07: Product and Brand Portfolio Analysis Module 08: Channels Management Module 09: Basics of Marketing for Products Module 10: Financial Analysis for Product Management Assessment Process You have to complete the assignment questions given at the end of the course and score a minimum of 60% to pass each exam. Our expert trainers will assess your assignment and give you feedback after you submit the assignment. You will be entitled to claim a certificate endorsed by the Quality Licence Scheme after you successfully pass the exams. CPD 220 CPD hours / points Accredited by CPD Quality Standards Who is this course for? The Bundle Course is ideal for: Professionals seeking to enhance their skills in product development and supply chain management. Individuals aspiring to enter the fields of production management, quality assurance, sales and marketing, and warehouse management. Business owners and entrepreneurs looking to optimise their product development and supply chain processes. Graduates or students interested in pursuing a career in product development or supply chain management. Career path This bundle/course will give you an edge in such professions: Product Development Manager (Average UK salary: £45,000 to £70,000 per year) Supply Chain Manager (Average UK salary: £40,000 to £75,000 per year) Production Manager (Average UK salary: £35,000 to £60,000 per year) Quality Assurance Manager (Average UK salary: £40,000 to £60,000 per year) Certificates Diploma in Product Development at QLS Level 4 Hard copy certificate - Included After successfully completing thecourse, you can order an original hardcopy Certificate of Achievement endorsed by the Quality Licence Scheme. This certificate will be delivered to your home, free of charge. To obtain the additional Quality Licence Scheme Endorsed Certificate, you will need to pay the corresponding fee based on the level of the courses: Level 1: £59 Level 2: £79 Level 3: £89 Level 4: £99 Level 5: £119 Level 6: £129 Level 7: £139 CPD QS Accredited Certificate Digital certificate - £10 Upon successfully completing the Bundle, you will need to place an order to receive a PDF Certificate for each course within the bundle. These certificates serve as proof of your newly acquired skills, accredited by CPD QS and will cost you £10 for each course. Also, the certificates are recognised throughout the UK and internationally. CPD QS Accredited Certificate Hard copy certificate - £29 International students are subject to a £10 delivery fee for their orders, based on their location.

Level 3 Procurement, Purchasing & Supply Chain Management Diploma
4.7(47)By Academy for Health and Fitness
24-Hour Knowledge Knockdown! Prices Reduced Like Never Before FREE PDF & Hard Copy Certificate| PDF Transcripts| FREE Student ID| Assessment| Lifetime Access| Enrolment Letter Ever wondered how goods get from factories to your doorstep? According to the Chartered Institute of Procurement & Supply (CIPS), the UK procurement and supply chain sector employs over 1.2 million people. This vast and ever-growing field offers exciting career opportunities for individuals with the right skills and knowledge. This Level 3 Procurement, Purchasing & Supply Chain Management Diploma bundle provides a structured learning pathway that equips you with the essential knowledge and skills needed to thrive in various roles within the procurement and supply chain sector. This training bundle will equip you with a thorough understanding of every step involved in getting a product from conception to the customer. You'll explore areas like strategic sourcing and international trade. By the course's end, you'll be able to identify cost-saving opportunities, streamline operations, and ensure quality throughout the supply chain. This Purchasing & Procurement at QLS Level 5 course is endorsed by The Quality Licence Scheme and accredited by CPD QS (with 150 CPD points) to make your skill development & career progression more accessible than ever! This Level 3 Procurement, Purchasing & Supply Chain Management Diploma Bundle Contains 20 of Our Premium Courses for One Discounted Price: Course 01: Diploma in Purchasing & Procurement at QLS Level 5 Course 02: Supply Chain Management Course 03: Material Management Course 04: Diploma in Operations Management Course 05: Logistics Management Course 06: Port Management Diploma Course 07: Transport and Logistics Management Course 08: Product Management Course 09: Lean Process and Six Sigma Training Course Course 10: Import/Export Processing Course 11: Cost Control Process and Management Course 12: Good Manufacturing Practices (GMP) Certification Course Course 13: Quality Assurance (QA) Manager Diploma Course 14: Purchase Ledger Training Course 15: Cost Control Process and Management Course 16: Retail Management Skills Course 17: Retail Assistant Course 18: Store Assistant Course 19: Manual Handling Training Course 20: Business Law Give your career a boost by enrolling today! Learn in-demand skills that are highly sought after by businesses across various industries. Learning Outcomes of Procurement, Purchasing & Supply Chain Management Gain a comprehensive understanding of procurement and purchasing processes. Develop expertise in logistics and supply chain management. Learn how to optimize inventory levels and reduce costs. Implement quality control measures to ensure product and service excellence. Master lean process and Six Sigma methodologies for continuous improvement. Gain the knowledge and skills required for a successful career in procurement, purchasing, or supply chain management. Why Choose Us? Get a Free CPD Accredited Certificate upon completion of Procurement Get a free student ID card with Procurement Training program (£10 postal charge will be applicable for international delivery) The Procurement is affordable and simple to understand This course is entirely online, interactive lesson with voiceover audio Get Lifetime access to the Procurement course materials The Procurement comes with 24/7 tutor support Start your learning journey straightaway! *** Course Curriculum *** Course 01: Diploma in Purchasing & Procurement at QLS Level 5 Module 01: Why Supply Chain Management Module 02: Three Levels of Supply Chain Management Module 03: Five Stages of Supply Chain Management Module 04: The Flows of Supply Chain Management Module 05: Supply Chain Groups Module 06: Tracking and Monitoring Module 07: Role of Procurement in an Organisation Module 08 : The Purchasing Cycle Module 09: Purchasing Toolkits Module 10: Quality Management Module 11: Supplier Relationship Management Module 12: Category Management Module 13: Inventory Management Module 14: E-Procurement Course 02: Supply Chain Management Module 01: An Overview Of Supply Chain Management Module 02: Role Of Logistics Of Supply Chain Module 03: Customer Response Principles And Systems Module 04: Supply Chain System And Principles Module 05: Global Supply Chain Operations Module 06:Inventory Strategies For Supply Chain Module 07: Warehouse Management And Operations Module 08: Supply Chain Design And Monitoring Module 09: Supply Chain Network And Evaluating Module 10: Supply Chain Planning And New Business Concepts Module 11: Aligning Your Supply Chain Process With Your Business Course 03: Material Management Module 01: Introduction To Material Management Module 02: Purchasing And Supply Chain Management Module 03: Inventory Management, Planning And Control Module 04: Stores Management And Control Module 05: Forecasting Module 06: Material Requirements Planning Module 07: Materials Handling =========>>>>> And 17 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*20 = £260) CPD Hard Copy Certificate: Free ( For The First Course: Previously it was £29.99) CPD 350 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Procurement and purchasing professionals. Supply chain management aspirants. Operations and logistics managers. Quality assurance and compliance officers. Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Procurement Manager Supply Chain Analyst Logistics Coordinator Quality Assurance Specialist Operations Manager Compliance Officer Certificates CPD Accredited Digital Certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. CPD Accredited Hard Copy Certificate Hard copy certificate - Included Please note that International students have to pay an additional £10 as a shipment fee. Diploma in Purchasing & Procurement at QLS Level 5 Hard copy certificate - £119 Please note that International students have to pay an additional £10 as a shipment fee.

CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

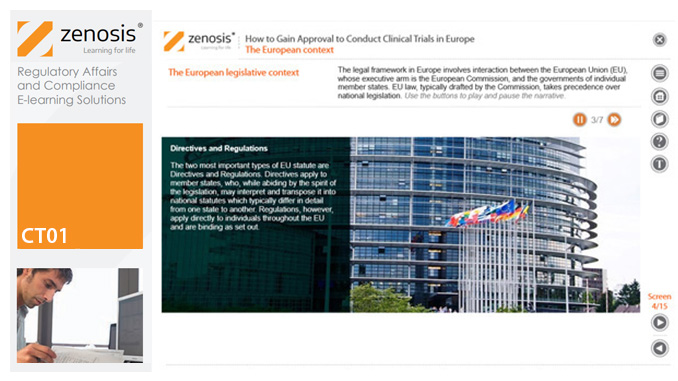
CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
By Zenosis
To conduct a clinical trial in the European Economic Area under the Clinical Trials Directive the sponsor must apply for authorisation from the national competent authority (i.e. medicines regulator), and favourable opinion must be obtained from a research ethics committee, in each member state in which the trial is to take place. This module sets out the requirements for successful compilation, submission and maintenance of the applications.
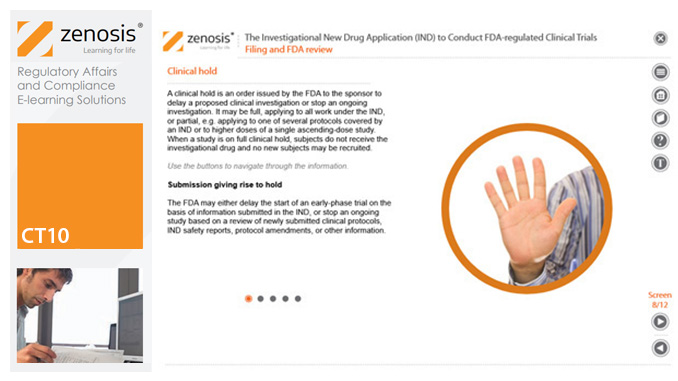
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
PV03: Drug Safety and Pharmacovigilance
By Zenosis
Drug safety monitoring and risk management are vitally important for medicinal product developers, licence holders and clinical investigators. In addition to their duty to protect public health, increasingly tight regulation and potentially massive payments to litigants provide strong incentives for pharmaceutical and biotechnology companies to ensure that they maintain efficient systems for drug safety / pharmacovigilance and that all staff are aware of the basic requirements. This course will provide them with an overview of the most important aspects of this discipline, both before and after marketing of products, especially as they apply in Europe and the USA.

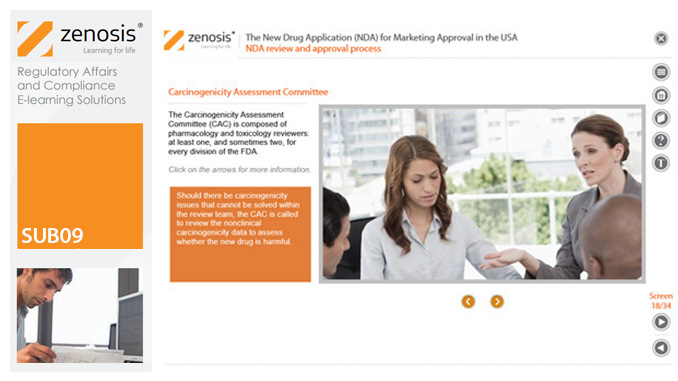
SUB09: The New Drug Application (NDA) for Marketing Approval in the USA
By Zenosis
The New Drug Application (NDA) is the regulatory vehicle through which sponsors formally propose that the Food and Drug Administration (FDA) approve a new pharmaceutical for marketing and sale in the USA.
CT04: An Introduction to Clinical Trial Preparation and Design
By Zenosis
This module aims to provide you with effective strategies for the preparation and conduct of a clinical trial, while adhering to regulatory safety standards. Management of data for submission is also covered.

SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

Search By Location
- Good Manufacturing Practice (GMP) Courses in London
- Good Manufacturing Practice (GMP) Courses in Birmingham
- Good Manufacturing Practice (GMP) Courses in Glasgow
- Good Manufacturing Practice (GMP) Courses in Liverpool
- Good Manufacturing Practice (GMP) Courses in Bristol
- Good Manufacturing Practice (GMP) Courses in Manchester
- Good Manufacturing Practice (GMP) Courses in Sheffield
- Good Manufacturing Practice (GMP) Courses in Leeds
- Good Manufacturing Practice (GMP) Courses in Edinburgh
- Good Manufacturing Practice (GMP) Courses in Leicester
- Good Manufacturing Practice (GMP) Courses in Coventry
- Good Manufacturing Practice (GMP) Courses in Bradford
- Good Manufacturing Practice (GMP) Courses in Cardiff
- Good Manufacturing Practice (GMP) Courses in Belfast
- Good Manufacturing Practice (GMP) Courses in Nottingham