- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
9685 ET courses
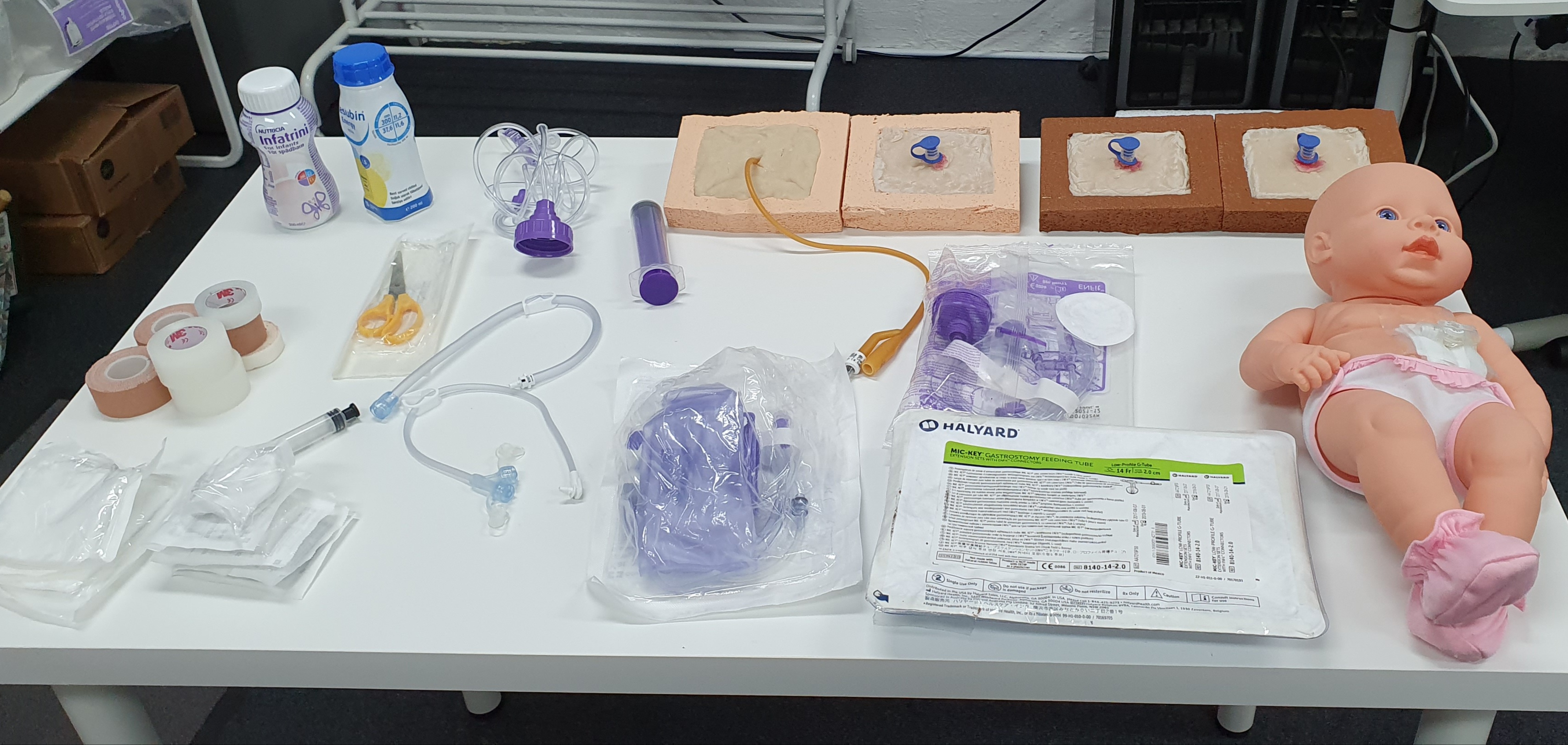
Gastrostomy tube care Gastrostomy tube management Gastrostomy tube training Enteral feeding care Gastrostomy tube complications Gastrostomy site care Gastrostomy tube insertion Gastrostomy tube feeding techniques Gastrostomy tube nursing Gastrostomy care for healthcare professionals Gastrostomy tube education CPD accredited course Nursing revalidation hours Practical gastrostomy care training Hands-on gastrostomy tube practice Patient education in gastrostomy care Ethical considerations in gastrostomy tube care Cultural sensitivity in gastrostomy care Gastrostomy tube complications prevention High-quality gastrostomy care certification PEG
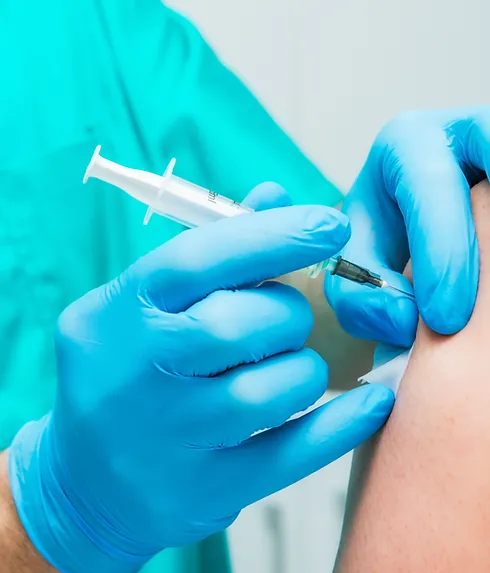
Learn how to administer vaccines or injections ... Nationally Recognised Qualification Includes IM, ID and Sub-Cut Injection methods OCN Accredited - Level 4 (Foundation Degree - FDSc) Covers all steps to safely perform a vaccination Use same techniques and skills for aesthetic therapies Includes B12, Vitamin C and other treatments Essential qualification for all injections Basic understanding of English language required OPEN TO ALL APPLICANTS
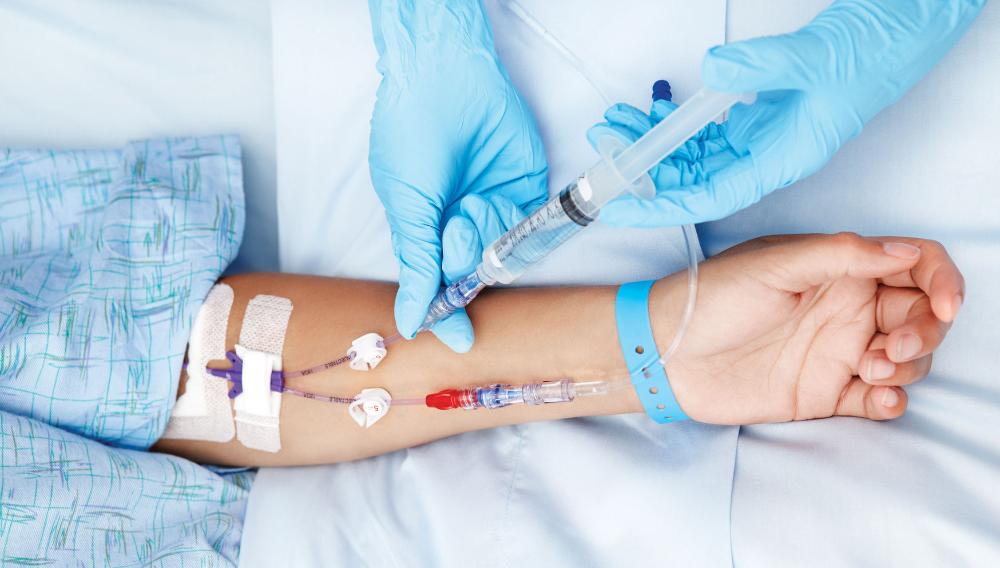
Learn how to cannulate ... Nationally Recognised Qualification OCN Accredited - Level 3 (advanced) - Ireland Level 5 CPD Accredited - The CPD Certification Service Classroom or Virtual Classroom options Covers all steps for cannulating in arm or hand Practise on artificial arms and fake blood! Essential qualification for all IV therapies Phlebotomy training desirable but not essential Basic understanding of English language required OPEN TO ALL APPLICANTS
Building Better Careers with Soft Skills: 1-Day Workshop in Milton Keynes
By Mangates
10 Soft Skills You Need 1 Day Training in Milton Keynes

Building Better Careers with Soft Skills: 1-Day Workshop in Slough
By Mangates
10 Soft Skills You Need 1 Day Training in Slough

Building Better Careers with Soft Skills: 1-Day Workshop in Windsor Town
By Mangates
10 Soft Skills You Need 1 Day Training in Windsor Town

Building Better Careers with Soft Skills: 1-Day Workshop in Craigavon
By Mangates
10 Soft Skills You Need 1 Day Training in Craigavon

Building Better Careers with Soft Skills: 1-Day Workshop in Burton Upon Trent
By Mangates
10 Soft Skills You Need 1 Day Training in Burton Upon Trent

Search By Location
- ET Courses in London
- ET Courses in Birmingham
- ET Courses in Glasgow
- ET Courses in Liverpool
- ET Courses in Bristol
- ET Courses in Manchester
- ET Courses in Sheffield
- ET Courses in Leeds
- ET Courses in Edinburgh
- ET Courses in Leicester
- ET Courses in Coventry
- ET Courses in Bradford
- ET Courses in Cardiff
- ET Courses in Belfast
- ET Courses in Nottingham

