- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
3121 Environment courses
AAT Level 1 Award in Business Skills
By London School of Science and Technology
The CAIA Association is a global professional body dedicated to creating greater alignment, transparency, and knowledge for all investors, with a specific emphasis on alternative investments. Course Overview At the start of any career, gaining a solid understanding of key business skills is extremely valuable. These qualifications and courses are designed to blend core skills with key finance knowledge, to give you essential business skills. This Level 1 qualification covers a range of skills and relevant supporting knowledge to help prepare students for applying numbers in business and working in a business environment. It will give students an understanding of: • How different organisations operate • How to contribute effectively in the workplace • How businesses process sales and purchases, and the documents and procedures associated with this. Students will also be equipped with the basic numerical skills needed in the workplace, such as decimals, percentages and fractions, and applying proportions and ratios. The jobs it can lead to: • Data entry clerk • Accounts administrator • Administrative assistant Entry requirements: Students can start with any qualification depending on existing skills and experience. For the best chance of success, we recommend that students begin their studies with a good standard of English and maths. Course Content: Working in the business environment: This unit introduces students to the skills needed in the workplace, including the importance of teamwork, communication, effective time management and professional behaviour. They’ll also learn about some of the core finance processes linked to sales and purchase orders. Learning outcomes: • Develop skills for the workplace. • Understand how organisations operate. • Understand how sales and purchases support businesses. • Apply business procedures to sales and purchases. Using numbers in business: Numeracy is an essential business skill. This unit will introduce students to the basic skills needed when working with numbers in a business environment, developing confidence and skills to use and apply numbers to a wide range of situations. Learning outcomes: • Perform simple business calculations. • Calculate decimals, fractions, percentage, proportions and ratios. • Use tools and techniques to present numerical data.

Managing Benefits Practitioner: In-House Training
By IIL Europe Ltd
Managing Benefits™ Practitioner: In-House Training Managing Benefits™ provides generally applicable guidance encompassing benefits management principles, practices, and techniques. The purpose of the Practitioner-level qualification is to confirm whether you have achieved sufficient understanding of how to apply and tailor the Managing Benefits guidance and, specifically, the principles, practices, techniques, roles, and documentation in a scenario situation. A Practitioner candidate should, with suitable support, be able to plan, implement, sustain, and measure benefits management while adapting to different organizational environments. You should also be able to: Identify activities that should be undertaken during each of the practices of the Benefits Management Cycle, together with the accountabilities and responsibilities of each of the defined roles Evaluate examples of benefits management information (documents) Analyse the solutions adopted in relation to a given scenario This course will prepare you to take and pass the Practitioner exam, which is held on the afternoon of the second day. Using APMG-provided sample exam papers and additional project case studies, you will learn how to apply what you've learned during the Foundation course and how to approach the scenario-based Practitioner exam, which is aimed at testing your ability to apply Managing Benefits in an actual project environment (via simulated case study). What You Will Learn You'll learn how to: Plan for the implementation of benefits management Select appropriate strategies to sustain and measure progress Select and adapt principles, practices, and techniques to suit different organizational environments Identify activities that should be undertaken during each of the practices of the Benefits Management Cycle together with the accountabilities and responsibilities of each of the defined roles Evaluate examples of benefits management information (documents) Analyse the solutions adopted in relation to a given scenario Improve your ability to pass the APMG Managing Benefits Practitioner Certification exam Introductions Course structure Course goals and objectives Overview and Principles Review Managing Benefits Practices Managing Benefits Practitioner Exam Preparation Practitioner Exam Briefing Review of and practice with APMG sample questions and test papers Sample project case study scenarios to apply the concepts in practice and deepen the learning Sample Practitioner Exam Debrief Results from Sample Practitioner Exam APMG Managing Benefits Practitioner Exam

A System Approach to Good Pharmacovigilance Practice
By Research Quality Association
Course Information In today's evolving landscape, pharmacovigilance (PV) systems face ongoing challenges due to global, national, and company-specific events. This course focuses on developing personnel equipped to navigate these complexities and improve the safe use of medicinal products. We emphasise continuous global thinking, communication, and strategic planning, ensuring adaptability across various levels. The course explores maintaining PV system functionality while adhering to regulatory requirements. Participants will apply European regulatory standards to enhance and sustain PV system effectiveness, contributing to improvement initiatives and ensuring operational integrity. The course covers PV system intricacies, regulatory compliance, and a 'systems approach' for auditors, quality assurance personnel, and PV practitioners. Through presentations and workshops, attendees gain insights into implementing and maintaining an effective PV system. Who should attend? Auditors Pharmacovigilance Quality System Managers Pharmacovigilance scientists The QPPV. Course benefits Throughout the course delegates will explore application of the legal requirements for the PV system and quality system and how to assure these systems. They will demonstrate their ability to contribute to: A systematic investigation of the pharmacovigilance system and its quality system Examination of how the pharmacovigilance system and quality system interact to achieve compliance. The risk-based approach to auditing the PV system and quality system The maintenance of 'inspection readiness' Explore how to investigate the complex PV system Discussions about how to monitor and maintain the PV system and assure compliance. Course Objectives Clarify what has to be done: Explore application of the legal requirements. Explore how to do what has to be done: Adopt a systemic approach to systematically investigate or implement and maintain the PV system and quality system Examine how a compliant PV system and a compliant quality system interact to achieve compliance with regulatory requirements for PV Explore how to investigate the complexity of the PV system. Discus how to identify what is missing or what needs to be improved: Discuss how to monitor and maintain the PV system and assure compliance. This course will assist delegates with: An understanding of key system principles, A practical approach to implementing, maintaining and monitoring the PV system and its quality system A procedure to share expertise to increase efficiency and confidence. This course is structured to encourage delegates to: Discuss and develop ideas - Share knowledge and experiences - Solve specific problems. By the end of the course delegates will be able to: Understand better the pharmacovigilance system, its quality system and how the components interact to achieve the objectives of pharmacovigilance Investigate, and analyse the pharmacovigilance system and to identify what is missing and what needs to be improved. Tutors Tutors will be comprised of (click the photos for biographies): Jana Hyankova Head of PV Department, IVIGEE Services a.s. Programme Please note timings may be subject to alteration. Day 1 08:30 Welcome, registration, course objectives and introduction to work groups Housekeeping notices, meet other delegates, explore how to work in your work group, course objectives. Clarify the definition and objectives of Pharmacovigilance. 09:30 The Regulatory Framework for Pharmacovigilance Identify the relevant regulations and directives. Explore GVP guidance, structure of the modules and standard format of each module. 10:00 The Pharmacovigilance System Exploration of how to organise what has to be done, communications. Exploration of the structures and processes for pharmacovigilance. 10:30 Break 11:00 Workshop 1 and Feedback Exploring an organisational model of the pharmaceutical company- cooperation between PV and other stakeholders. 12:00 The Quality System for pharmacovigilance Exploration of the structures, processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 13:00 Lunch 14:00 Workshop 2 and Feedback The quality system puzzle. Explore the organisation of the PV quality system and how it interacts with the PV system. 14:30 The Quality System for pharmacovigilance Exploration of the structures processes for the PV quality system and discussion of how it interacts with the pharmacovigilance system to meet the objectives of pharmacovigilance effectively and efficiently. 15:00 Description of PV System 15:30 Break 15:30 Workshop 3 and Feedback The quality system puzzle Explore the organisation of the PV quality system and how it interacts with the PV system. 16:00 The Pharmacovigilance Safety Master File Construction of the Pharmacovigilance System Master File and its purpose. 17:00 Workshop 3 and Feedback Description of PV System. 18:00 End of Day Day 2 08:30 Drug Safety in the Clinical Trial Environment - Part 1 Information flow and responsibilities of the sponsor. 09:30 Workshop 4 and Feedback Drug Safety in the Clinical Trial environment: Information flow and responsibilities of the sponsor. 10:30 Break 11:00 Drug Safety in the Clinical Trial Environment - Part 2 Information flow and responsibilities of the sponsor. 12:00 Lunch 13:00 Workshop 5 and Feedback Drug Safety in the clinical trial environment: Information flow and responsibilities of the sponsor. 13:30 Processing of Safety Data Exploration of safety data processing, verification, validation, follow up, formatting and collation, reporting requirements, quality and data management. 15:00 Break 15:30 EudraVigilance Exploration of how EudraVigilance supports the PV system. 16:15 Signal Detection and Evaluation/Risk Benefit Assessment: Pharmacovigilance Risk Assessment Committee (PRAC): What is a signal? What are the regulatory requirements? How is signal detection and evaluation conducted? Qualitative and quantitative methods of signal detection. Risk benefit assessment. 17:00 Risk Management Plans A cornerstone of Pharmacovigilance safety communications, direct healthcare professional communication 18:00 End of Day Day 3 08:30 The Pharmacovigilance Risk Assessment Committee (PRAC) Exploration of how good practice is achieved. Composition, role and responsibilities. Examples of referrals. 09:15 Development Safety Update Reports (DSURs): Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 10:30 Break 11:00 Periodic Safety Update Reports (PSURs)/Periodic Benefit Risk Evaluation Reports (PBRERs) Regulatory requirements, exploring good practice, report format, reference safety information, schedule of submission, analysis evaluations and distribution. 12:00 Workshop 6 and Feedback To explore the compilation and submission of the PSUR. 13:00 Lunch 13:30 Role of the QPPV Exploration of the legal responsibilities of the QPPV and the MAH. 14:30 Break 15:00 Workshop 7 and Feedback To explore the challenges faced by the QPPV. 15:30 End of course Extra Information Face-to-Face Course Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device< Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam. CPD Points 23 Points Development Level Develop

SAFe for Government
By IIL Europe Ltd
SAFe® for Government Transitioning to Lean-Agile practices for building technology-based capabilities is especially challenging in the government context. But issues of legacy governance, contracting, and organizational barriers can be overcome with the right information and strategies. During this course, attendees will learn the principles and practices of the Scaled Agile Framework® (SAFe®), how to execute and release value through Agile Release Trains, and what it means to lead a Lean-Agile transformation of a program inside a government agency. Attendees gain an understanding of the Lean-Agile mindset and why it's an essential foundation for transformation. They'll also get practical advice on building high-performing, multi-vendor Agile teams and programs, managing technology investments in Lean flow, acquiring solutions with Agile contracting, launching the program, and planning and delivering value using SAFe®. Attendees also learn how specific leadership behaviors can drive successful organizational change in government. What you will Learn To perform the role of a SAFe® for Government leader, you should be able to: Transition government programs from traditional software and systems development models to Lean-Agile and DevOps mindsets, principles, and practices using SAFe® Adapt technology strategy, budgeting and forecasting, acquisition, compliance, and governance processes to flow-based practices using emerging government guidelines Organize government programs into one or more Agile Release Trains (ARTs) and execute in Program Increments (PIs) Explore Large Solution coordination in a government and multi-vendor environment Identify and internalize the mindset and leader behaviors essential to successful Lean-Agile transformation Follow success patterns for SAFe® implementations adapted to the government context Build a preliminary outline of next steps to begin and / or accelerate the SAFe® implementation in your program or agency Advancing Lean-Agile in government Embracing a Lean-Agile mindset Understanding SAFe® Principles Creating high-performing Agile teams and programs Planning with cadence and synchronization Delivering value in Program Increments Mapping the path to agency and program agility Leading successful change

M.D.D COACHING FOR CEOS PACKAGE (SELF IMPROVEMENT)
4.9(27)By Miss Date Doctor Dating Coach London, Couples Therapy
Solution-Focused Coaching Model Grow Coaching Model 0SKAR Coaching Model CLEAR Coaching Model AOR Coaching Model FUEL Coaching Model WOOP Coaching Model COACHING PROCEDURE FOR COACHING FOR CEOS: Pre-session check-in and questionnaire and assessment Mindset Analysis and introspection testing Psychological analysis Follow up for feedback and well-being and progress check-up calls Accountability Coaching Anxiety Coaching Target-driven coaching helpful for individuals with procrastination issues or issues tackling challenging career goals Leadership Coaching and Self-belief Coaching Organizational objective coaching Bring awareness to self-talk and unconscious biases 24/7 ACCESS TO YOUR COACH Coach available on Whatsapp, Phone, Zoom, and Face to Face CEO Coaching helps coach the world’s best CEOs, executives, entrepreneurs, and companies to increase revenue and grow their business. Our coaches have extensive real-world experience in overcoming the challenges, inherent in entrepreneurship and executive leadership. Consult with a CEO coach today to receive a customized business plan that will enable you to achieve extraordinary results. We work with you by understanding the business issues and strategic imperatives of the enterprise. We then use a combination of 360 feedback (including Board members) and formal assessment tools to ensure we have a clear picture of you as an individual leader and the environment in which you need to create success. We assess your team and work with you to understand where you’ve got the capability and where the team may be vulnerable. https://relationshipsmdd.com/product/coaching-for-ceos-package/

ILM Level 2 – Award in Customer Care
By Challenge Consulting
ILM Level 2 Award in Customer Care – 4 day Accredited training course delivered in Nottingham A nationally recognised qualification taught across 4 x 1 day deliveries approximately 3 weeks apart. The course is specifically tailored for individuals whose job role requires direct customer interface, and where influencing and meeting the needs of customers is particularly important. The course brings tangible benefits to the participants and to their organisation through applying concepts taught at each stage of the course directly to the work environment, and providing opportunities to compare best practice methods and techniques with current practices.

Permanent Makeup Laser Removal | N-Light-10 Laser Only
By ID Liner | Permanent Makeup Training & Supplies
HIGHLY SOUGHT-AFTER AND ALREADY AN EXTREMELY LUCRATIVE INDUSTRY, LASER TATTOO REMOVAL IS SET TO BE THE BIGGEST BEAUTY TREND OF 2023!

Permanent Makeup Laser Removal - Small Group Training - N-Light-10 Laser
By ID Liner | Permanent Makeup Training & Supplies
HIGHLY SOUGHT-AFTER AND ALREADY AN EXTREMELY LUCRATIVE INDUSTRY, LASER TATTOO REMOVAL IS SET TO BE THE BIGGEST BEAUTY TREND OF 2023!

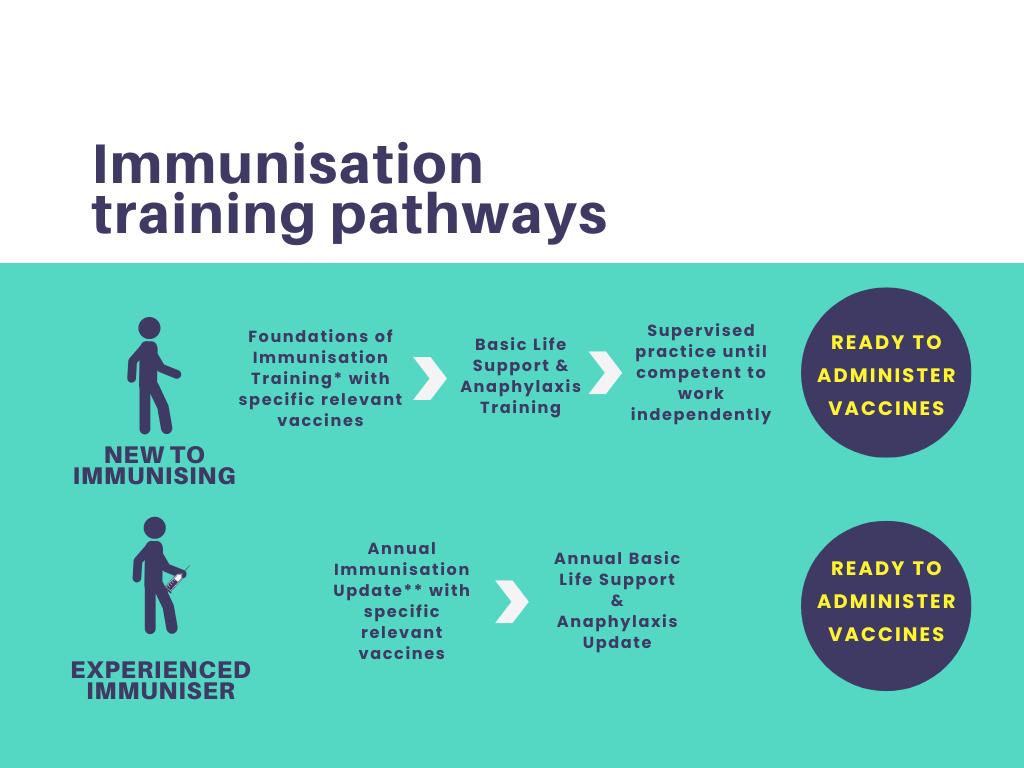
Foundations of Immunisation and Vaccines for Non-Registered Practitioners
By Guardian Angels Training
The "Foundations of Immunisation and Vaccines for Non-Registered Practitioners" course is fully compliant with the National Minimum Standards and Core Curriculum for Immunisation Training and is designed to equip non-registered healthcare professionals with a solid understanding of immunisation concepts, vaccine administration, and the importance of vaccination in public health.
SAFe for Government: In-House Training
By IIL Europe Ltd
SAFe® for Government: In-House Training Transitioning to Lean-Agile practices for building technology-based capabilities is especially challenging in the government context. But issues of legacy governance, contracting, and organizational barriers can be overcome with the right information and strategies. During this course, attendees will learn the principles and practices of the Scaled Agile Framework® (SAFe®), how to execute and release value through Agile Release Trains, and what it means to lead a Lean-Agile transformation of a program inside a government agency. Attendees gain an understanding of the Lean-Agile mindset and why it's an essential foundation for transformation. They'll also get practical advice on building high-performing, multi-vendor Agile teams and programs, managing technology investments in Lean flow, acquiring solutions with Agile contracting, launching the program, and planning and delivering value using SAFe®. Attendees also learn how specific leadership behaviors can drive successful organizational change in government. What you will Learn To perform the role of a SAFe® for Government leader, you should be able to: Transition government programs from traditional software and systems development models to Lean-Agile and DevOps mindsets, principles, and practices using SAFe® Adapt technology strategy, budgeting and forecasting, acquisition, compliance, and governance processes to flow-based practices using emerging government guidelines Organize government programs into one or more Agile Release Trains (ARTs) and execute in Program Increments (PIs) Explore Large Solution coordination in a government and multi-vendor environment Identify and internalize the mindset and leader behaviors essential to successful Lean-Agile transformation Follow success patterns for SAFe® implementations adapted to the government context Build a preliminary outline of next steps to begin and / or accelerate the SAFe® implementation in your program or agency Advancing Lean-Agile in government Embracing a Lean-Agile mindset Understanding SAFe® Principles Creating high-performing Agile teams and programs Planning with cadence and synchronization Delivering value in Program Increments Mapping the path to agency and program agility Leading successful change

Search By Location
- Environment Courses in London
- Environment Courses in Birmingham
- Environment Courses in Glasgow
- Environment Courses in Liverpool
- Environment Courses in Bristol
- Environment Courses in Manchester
- Environment Courses in Sheffield
- Environment Courses in Leeds
- Environment Courses in Edinburgh
- Environment Courses in Leicester
- Environment Courses in Coventry
- Environment Courses in Bradford
- Environment Courses in Cardiff
- Environment Courses in Belfast
- Environment Courses in Nottingham