- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
101 Drug Safety courses
Dive into the core of healthcare safety with our comprehensive course, 'Safety & Precautions in Medication Management.' This journey begins with unraveling the basics of medicine handling, ensuring you develop a robust foundation in the art and science of pharmaceutical care. As we progress, you'll master the delicate art of safe medication handling and become proficient in the aseptic technique, emphasizing the paramount importance of health and safety management. In the anatomical exploration of the body's intricacies, you'll gain insights into how medications interact with different physiological systems. Hygiene and cleanliness take center stage as we highlight their pivotal role in maintaining health standards. We cap this enlightening voyage by immersing ourselves in the gold standard of infection control-standard precautions. Learning Outcomes Grasp the fundamentals of medicine handling for a strong foundation. Master the art of safe medication handling with precision and care. Develop expertise in aseptic techniques and health & safety management. Gain insights into the anatomy and physiology of crucial body parts. Understand the significance of hygiene, cleanliness, and standard precautions for infection control. Why choose this Safety & Precautions in Medication Management course? Unlimited access to the course for a lifetime. Opportunity to earn a certificate accredited by the CPD Quality Standards and CIQ after completing this course. Structured lesson planning in line with industry standards. Immerse yourself in innovative and captivating course materials and activities. Assessments designed to evaluate advanced cognitive abilities and skill proficiency. Flexibility to complete the Course at your own pace, on your own schedule. Receive full tutor support throughout the week, from Monday to Friday, to enhance your learning experience. Unlock career resources for CV improvement, interview readiness, and job success. Who is this Safety & Precautions in Medication Management course for? Healthcare professionals aiming to enhance their medication management skills. Aspiring pharmacists seeking a comprehensive understanding of safe medication practices. Caregivers and support staff involved in medication administration. Individuals keen on bolstering their knowledge of health and safety in a medical context. Anyone interested in fostering a safe and secure healthcare environment. Career path Medication Safety Officer: £28,000 - £38,000 Pharmaceutical Care Specialist: £30,000 - £40,000 Healthcare Compliance Manager: £35,000 - £45,000 Clinical Risk Analyst: £25,000 - £35,000 Infection Control Practitioner: £28,000 - £38,000 Patient Safety Coordinator: £30,000 - £40,000 Prerequisites This Safety & Precautions in Medication Management does not require you to have any prior qualifications or experience. You can just enrol and start learning.This Safety & Precautions in Medication Management was made by professionals and it is compatible with all PC's, Mac's, tablets and smartphones. You will be able to access the course from anywhere at any time as long as you have a good enough internet connection. Certification After studying the course materials, there will be a written assignment test which you can take at the end of the course. After successfully passing the test you will be able to claim the pdf certificate for £4.99 Original Hard Copy certificates need to be ordered at an additional cost of £8. Course Curriculum Module 01: Basics of Medicine Handling Basics of Medicine Handling 00:15:00 Module 02: Safe Handling of Medications Safe Handling of Medications 00:15:00 Module 03: Aseptic Technique and Health & Safety Management Aseptic Technique and Health & Safety Management 00:17:00 Module 04: Anatomy and physiology of Body Parts Anatomy and physiology of Body Parts 00:16:00 Module 05: Importance Hygiene & Cleanliness Importance Hygiene & Cleanliness 00:15:00 Module 06: Standard Precautions for Infection Control Standard Precautions for Infection Control 00:17:00

CT03: ICH Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data. The International Council for Harmonisation’s guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice.

CT01: How to Gain and Maintain Approval for Clinical Research Under the EU Clinical Trials Directive
By Zenosis
To conduct a clinical trial in the European Economic Area under the Clinical Trials Directive the sponsor must apply for authorisation from the national competent authority (i.e. medicines regulator), and favourable opinion must be obtained from a research ethics committee, in each member state in which the trial is to take place. This module sets out the requirements for successful compilation, submission and maintenance of the applications.
CT10: The Investigational New Drug Application (IND) to Conduct FDA-regulated Clinical Trials
By Zenosis
An Investigational New Drug Application (IND) is a submission to the US Food and Drug Administration (FDA) for permission to conduct a clinical trial of a medicinal product. This module describes regulatory requirements that sponsors or sponsor-investigators must meet for successful compilation, filing and maintenance of INDs. The IND and its role are defined, and the contexts in which it is required are specified.
SUB09: The New Drug Application (NDA) for Marketing Approval in the USA
By Zenosis
The New Drug Application (NDA) is the regulatory vehicle through which sponsors formally propose that the Food and Drug Administration (FDA) approve a new pharmaceutical for marketing and sale in the USA.
CT04: An Introduction to Clinical Trial Preparation and Design
By Zenosis
This module aims to provide you with effective strategies for the preparation and conduct of a clinical trial, while adhering to regulatory safety standards. Management of data for submission is also covered.

SUB05: Electronic Common Technical Document (eCTD)
By Zenosis
The eCTD is mandatory for all applications for marketing approval and all subsequent related submissions in the European Economic Area, the USA and Canada. Other countries intend to make its use mandatory. The eCTD specification has been developed to facilitate the global electronic submission, review and lifecycle management of medicinal product dossiers for regulatory applications. It broadens the scope of the CTD to include information on variations, renewals and amendments, so that it is no longer a static document but is updatable throughout the life of the product. This module outlines the eCTD specification, discusses the approach to regional differences in dossiers, and provides guidance on creation of an eCTD submission. The module provides a training and reference tool that will be of particular value to those new to the use of the format.

CT09: Good Clinical Practice Inspections and Audits
By Zenosis
The module describes general principles of GCP inspection and audit, discusses preparation for an inspection, and sets out in detail what European and US FDA inspectors will examine. Finally it describes post-inspection actions by the regulator and the inspected party.

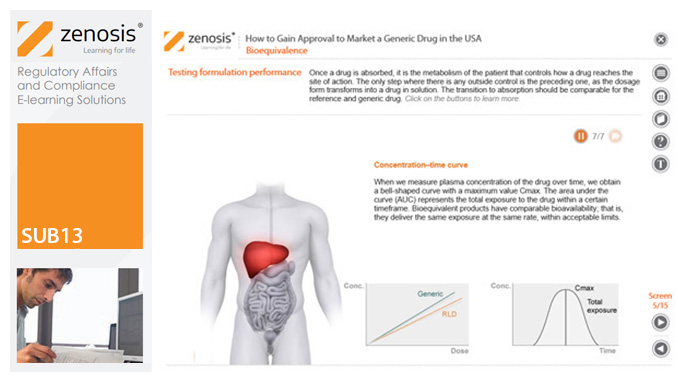
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
SUB14: The Regulatory Pathway to Licensure of Follow-on Biologics (Biosimilars) in the USA
By Zenosis
The regulation of biological medicinal products is governed by different laws from those that apply to small-molecule synthetic drugs. Producing faithful copies of therapeutic proteins is more challenging than producing generic drugs. The US legal framework for the licensure of follow-on biologics, and accompanying regulatory guidance from the Food and Drug Administration (FDA), have been established only in recent years.

Search By Location
- Drug Safety Courses in London
- Drug Safety Courses in Birmingham
- Drug Safety Courses in Glasgow
- Drug Safety Courses in Liverpool
- Drug Safety Courses in Bristol
- Drug Safety Courses in Manchester
- Drug Safety Courses in Sheffield
- Drug Safety Courses in Leeds
- Drug Safety Courses in Edinburgh
- Drug Safety Courses in Leicester
- Drug Safety Courses in Coventry
- Drug Safety Courses in Bradford
- Drug Safety Courses in Cardiff
- Drug Safety Courses in Belfast
- Drug Safety Courses in Nottingham