- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
10983 Courses delivered On Demand
Diploma in Leadership & Management
By Training Tale
***Diploma in Leadership & Management*** This Level 7 Leadership & Management course will help you advance your career by providing you with the most up-to-date leadership and management information. The course requires no prior knowledge or experience, as candidates are introduced to the characteristics and qualities of an effective leader. The term 'leader' refers to someone who is followed by others, while 'manager' refers to someone who has people working for them. To guide his or her team to achieve organizational goals, a successful business owner must be both an influential leader and an active manager. Whether you are a novice or a seasoned pro, this Training Tale course will help you develop the leader within you. Learn how to build and optimize a high-performing team, cultivate an engaging organizational culture, and provide constructive feedback to coworkers by enrolling in this course. This Level 7 Leadership & Management course will help you get closer to your goals if you are new to leadership or looking to advance in your current position! By the end of the Leadership & Management course, you will have a solid understanding of what makes a great leader and how to put the strategies you have learned into practice to advance your career. Enrol in this Course to learn how to improve your leadership and management skills. Learning Outcomes After completing this Leadership & Management Course you will be able to: Become a successful leader. Distinguish between management and leadership and their impact. Encourage and reward workers who increase their production. Improve your professional communication skills. Examine various approaches, causes, and tactics that can help you advance your leadership skills and capabilities. Define the terms 'delegation' and 'leading by example.' Improve your team-building abilities. Give and receive positive reviews. Improve interviewing skills for effective employee recruitment. Why Choose Diploma in Leadership & Management Course from Us Self-paced course, access available from anywhere. Easy to understand, high-quality study materials of Leadership & Management Course. Leadership & Management Course developed by industry experts. Leadership & Management Course MCQ quiz after each module to assess your learning. Leadership & Management CourseAutomated and instant assessment results. 24/7 support via live chat, phone call or email. Free PDF certificate as soon as completing the Leadership & Management Course course. ***Diploma in Leadership & Management*** Course 01: Diploma in Leadership & Management Course 02: Level 2 Diploma in Business Administration Course 03: Level 3 Business Administration ***Other Benefits of Diploma in Leadership & Management Free 3 PDF Certificate Access to Content - Lifetime Exam Fee - Totally Free Free Retake Exam [ Note: Free PDF certificate as soon as completing the course ] Detailed course curriculum Module 1: Understanding Management & Leadership Module 2: Leadership over Yourself Module 3: Creativity and Innovation Module 4: Leadership and Teambuilding Module 5: Motivation and People Management Module 6: Communication and Leadership Module 7: Presentation, One-to-one Interview and Meeting Management Module 8: Talent Management Module 9: Strategic Leadership Module 10: Stress Management ---------------------------- Assessment Method After you have finished the Diploma in Leadership & Management Course, you will need to take an electronic multiple-choice exam or Assignment to see if you have grasped everything. To pass the exam and be eligible for the pending certificates, you must achieve at least 60%. As soon as you pass the examination, you will be qualified to request your certification. Certification After completing the MCQ/Assignment assessment for this Leadership & Management course, you will be entitled to a Certificate of Completion from Training Tale. It will act as proof of your extensive professional development. The certificate is in PDF format, which is completely free to download. A printed version is also available upon request. It will also be sent to you through a courier for £13.99. Who is this course for? This Leadership and Management Course is for anyone serious about their professional development. This Leadership and Management Course is designed primarily for group leaders, managers at all levels, and business professionals. Students and recent graduates who want to improve their resumes and gain experience are also welcome to enrol in this Leadership and Management Course. Requirements There are no specific requirements for this Leadership and Management Course because it does not require any advanced knowledge or skills. Students who intend to enrol in this Course must meet the following requirements: Good command of the English language Must be vivacious and self-driven Basic computer knowledge A minimum of 16 years of age is required Certificates Certificate of completion Digital certificate - Included

VAL01: Introduction to Validation
By Zenosis
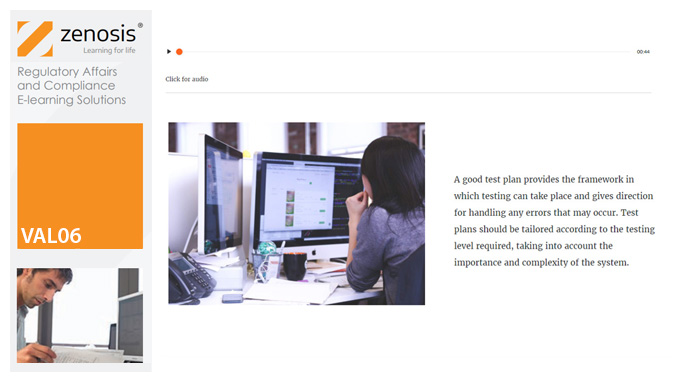
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL03: Commissioning and Installation Qualification
By Zenosis
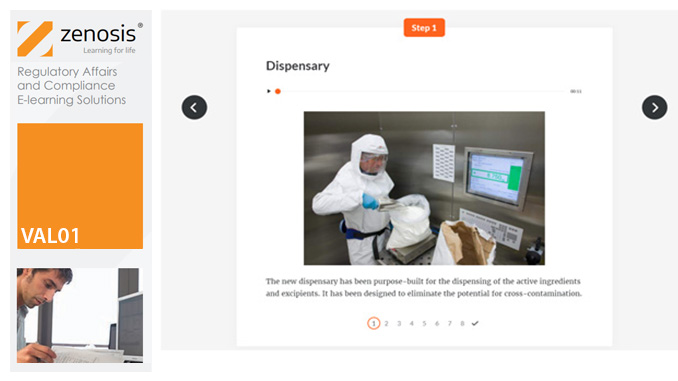
Before equipment can be used routinely in production, it must first be commissioned and, if necessary, undergo Installation Qualification (IQ). This module describes commissioning and IQ requirements and procedures in the medicines and healthcare products industries. It follows the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL02: Validation Plans and Documentation
By Zenosis
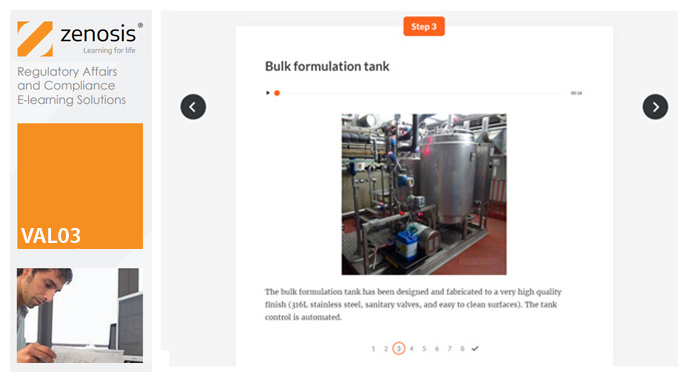
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
VAL05: Equipment Cleaning Validation
By Zenosis
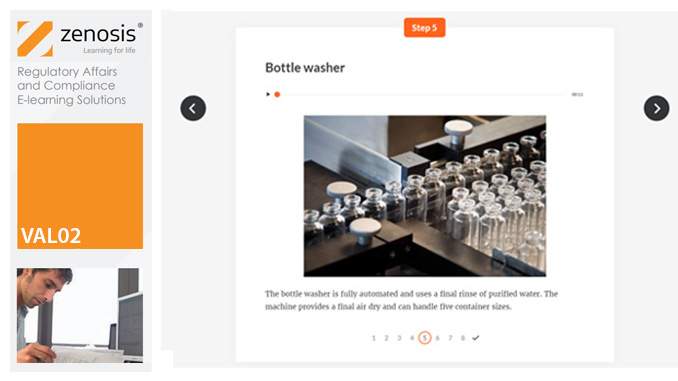
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
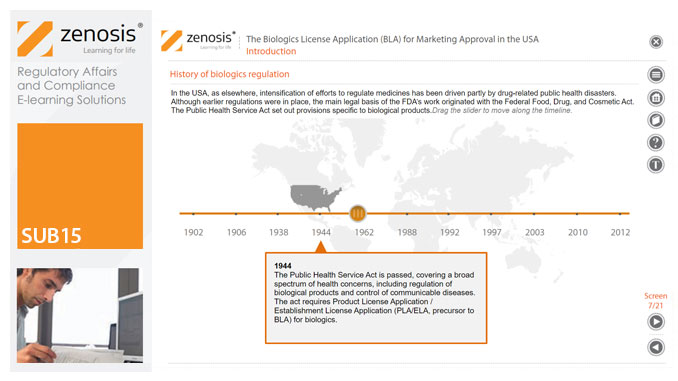
SUB15: The Biologics License Application (BLA) for Marketing Approval in the USA
By Zenosis
This module describes the requirements that must be met to obtain licensure of a biological product. Subjects covered include the regulatory context, the content and format of the BLA submission, the review process, and provisions for expedited development and review.
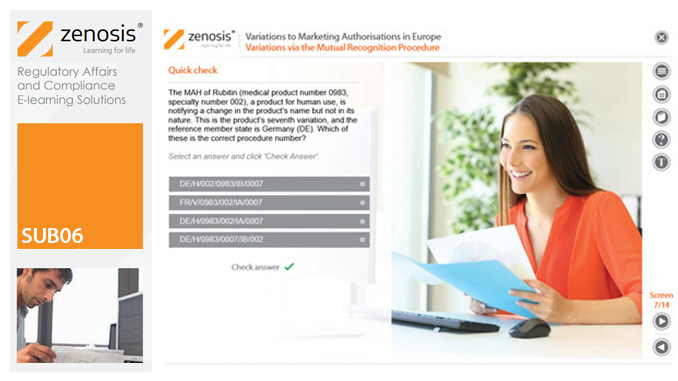
SUB06: Variations to Marketing Authorisations in Europe
By Zenosis
Changes to the terms of marketing authorisations for medicinal products, called variations in Europe, must be notified to or approved by the relevant regulatory authorities. Variations include changes to the composition of products, their manufacturing processes, the way they are used, or the indications for which they are authorised. Common approaches are adopted within the European Economic Area to variations to marketing authorisations approved through the Centralised, Decentralised or Mutual Recognition Procedures. Recent legislation has substantially modified the regulatory requirements and extended them to purely national authorisations by member states. This module, which is fully up to date with the new legislation, covers the classification of variations into their several types and the regulatory requirements, guidance and procedures to be followed for each type.
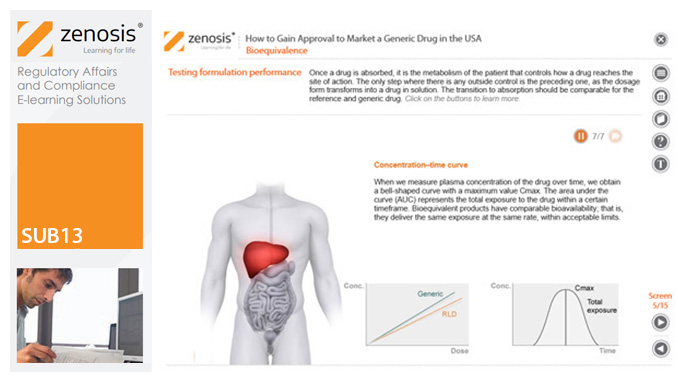
SUB13: How to Gain Approval to Market a Generic Drug in the USA
By Zenosis
This module outlines the legislative and regulatory context for the development of generic drugs and describes the essential role of the Abbreviated New Drug Application (ANDA) in gaining marketing approval. The use of information in the ‘Orange Book’ is explained, as is the role of patent certification in the application. The importance of establishing bioequivalence between a generic and its reference product is emphasised. The module specifies the content and format requirements for an ANDA submission and describes the FDA’s review and approval process. An outline is given of the Generic Drug User Fee Amendments (GDUFA) and the law’s effects on industry players.
ESS01: Essentials of EU and US Regulatory Affairs for Human Medicinal Products
By Zenosis
This foundation-level module is the ideal introduction for new entrants to the field of pharmaceutical regulatory affairs and compliance. It describes the principal requirements that must be satisfied to gain and maintain approval to market medicinal products in the USA and Europe. The legal framework and the roles of major players in regulation are presented. The life-cycle of a drug is outlined. The various procedures available for assessment and approval of products are described and their requirements outlined. Obligations to be fulfilled after marketing approval are discussed.

VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.