- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
All of us have struggled to decipher the long list of ingredients and recommendations on our food labels. How do we leverage this important information? This course is designed to give you a deeper understanding of labeling standards, along with tips and tricks for estimating healthy portion sizes. It was created by the National Academy of Sports Medicine (NASM), which for over 30 years has built its reputation on evidence-based recommendations that adhere to rigorous scientific standards..

There are literally thousands of diets out there. Some are based on science, but others are mostly hype. That’s why it’s so important for us to be well-informed with the latest facts regarding diets and how they impact our health and quality of life. This course explores many different diets and it reveals their various levels of safety and effectiveness. It was created by the National Academy of Sports Medicine (NASM), which for over 30 years has built its reputation on evidence-based recommendations that adhere to rigorous scientific standards. More than 20 experts in nutrition, psychology and behavior change were consulted to provide the most reliable guidance for people of varying ages, ethnicities, backgrounds, food preferences, and goals.

No matter how hard you try, there is no escaping the endless stream of advertising for all the nutritional supplements out there. From protein and creatine, to caffeine and other more-controversial performance aids, there is something tailored to every health, wellness, and fitness goal. Since each one claims to be the best and most effective, how do you separate research-supported hope from marketing hype? This course will help you make sense of the common trends in supplements. It was created by the National Academy of Sports Medicine (NASM), which for over 30 years has built its reputation on evidence-based recommendations that adhere to rigorous scientific standards. More than 20 experts in nutrition, psychology and behavior change were consulted to provide the most reliable guidance for people of varying ages, ethnicities, backgrounds, food preferences, and goals.

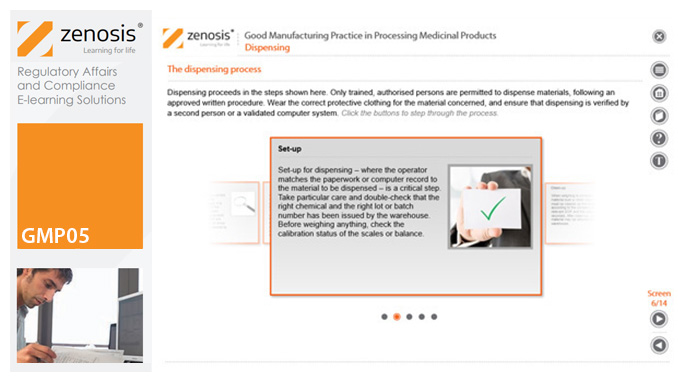
GMP05: Good Manufacturing Practice in Processing Medicinal Products
By Zenosis
Operations in the dispensary and on processing lines are at the heart of medicinal product manufacturing. This module describes how to carry out such operations in compliance with the requirements of Good Manufacturing Practice.
PV07: Good Pharmacoepidemiology Practice
By Zenosis
Pharmacoepidemiology is the study of the use and effects of drugs in large numbers of people. It provides a bridge between clinical pharmacology and epidemiology. The increasing demand for real-world evidence of the safety, efficacy and utility of medicinal products has focused greater attention on pharmacoepidemiological research. This module will help those who plan and conduct such research, and analyse and report the findings, to follow good practice.

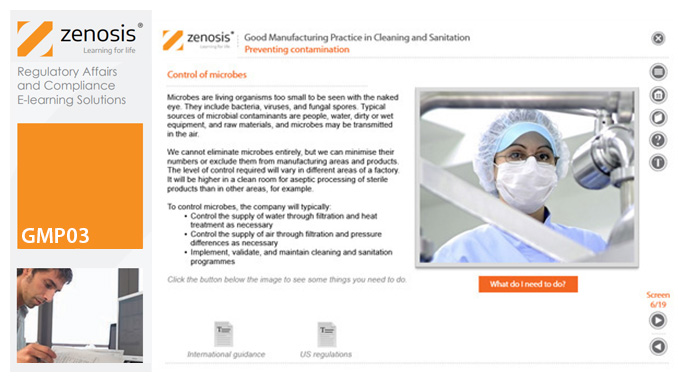
GMP03: Good Manufacturing Practice in Cleaning and Sanitation
By Zenosis
Cleaning and sanitation of premises and equipment are essential to efforts to prevent contamination of product, and they need to be done in compliance with Good Manufacturing Practice (GMP) regulatory requirements. This module shows why it is so important to do a good job, what to consider before and during each job, and how best to go about the work.
GMP06: Good Manufacturing Practice in Packaging Medicinal Products
By Zenosis
Packaging for medicinal products is subject to Good Manufacturing Practice rules similar to those for the products themselves. In this module we describe the functions that packaging must fulfil and the quality controls that are applied to packaging materials and operations. We set out the requirements for control of printed materials. We describe preparation, in-process control, and completion of a packaging run. Finally, we explain how to carry out reconciliation of packaging materials.

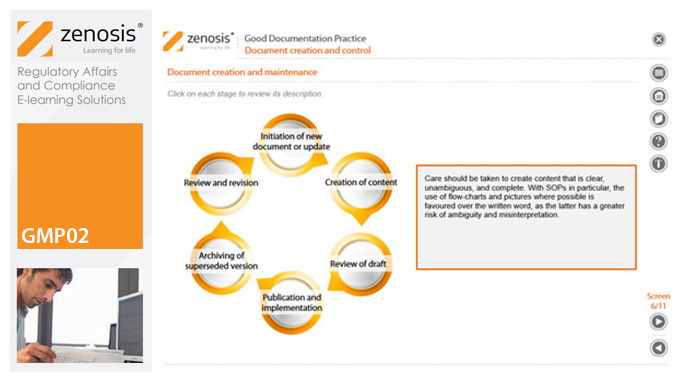
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
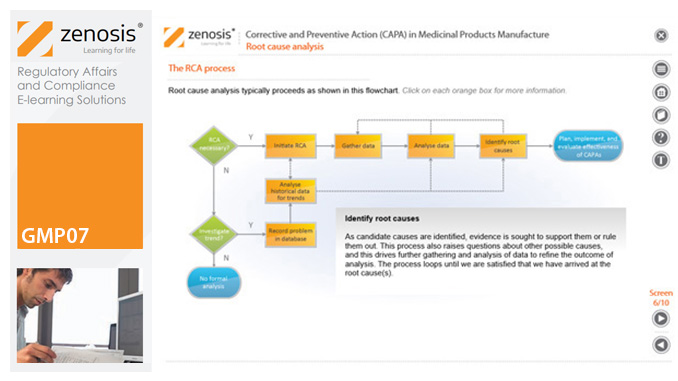
GMP07: Corrective and Preventive Action (CAPA) in Medicinal Products Manufacture
By Zenosis
A company’s Corrective and Preventive Action (CAPA ) system establishes how personnel should deal with manufacturing problems that have occurred or that may occur if not prevented. This module explains the principles of corrective and preventive action and describes typical CAPA procedure. It goes on to introduce root cause analysis and outline the role of progress tracking, escalating, and trending of CAPA procedures.
ESS02: Essentials of Monoclonal Antibodies
By Zenosis
This module will introduce you to monoclonal antibodies, explaining how they work, how they are made, and the many uses to which they are put.
