- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
388 Courses in Glasgow delivered On Demand
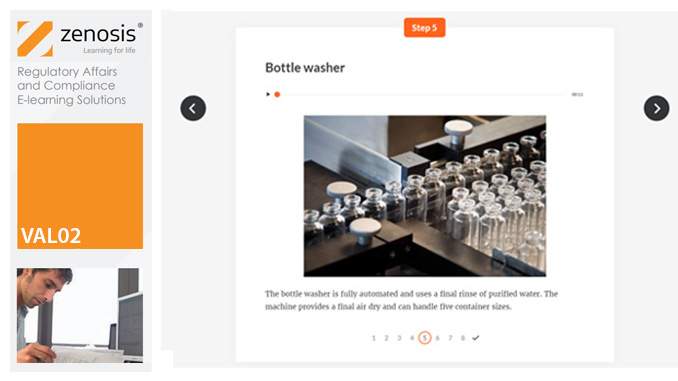
VAL02: Validation Plans and Documentation
By Zenosis
Essential to validation is the provision of documented evidence verifying that manufacturing processes will consistently result in products meeting predetermined quality standards. This module describes the purpose, content and use of validation master plans, project validation plans, and other documentation for validation projects in the medicines and healthcare products industries. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Pharmaceutical Validation Training Course (ONLINE) – For More Senior Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

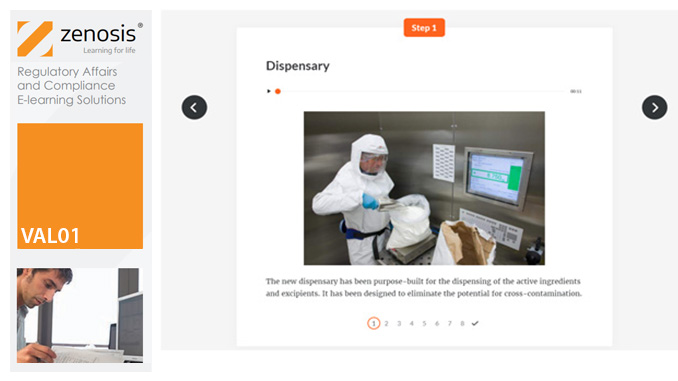
VAL01: Introduction to Validation
By Zenosis
Validation of equipment, services, systems and processes is vitally important in the medicines and healthcare products industries. Regulatory authorities require documented evidence that manufacturing processes will consistently result in products meeting predetermined quality standards. This module provides an introduction to validation and to the regulations and guidance that apply to it. It describes the activities of a typical validation team as they carry out a project for a pharmaceutical company.
Equipment Validation Training Course (ONLINE) – For Starter Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

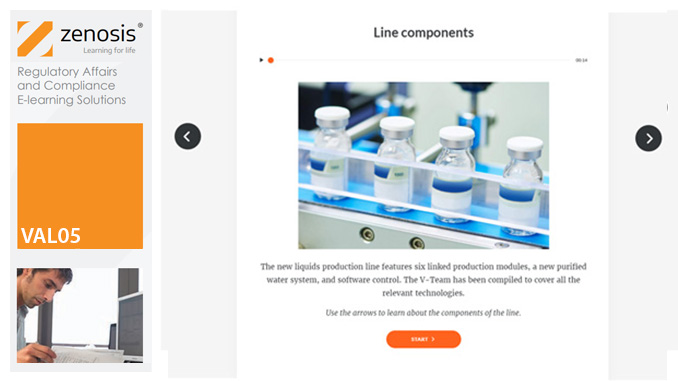
VAL05: Equipment Cleaning Validation
By Zenosis
Manufacturers of medicines and healthcare products must establish, validate and maintain an equipment cleaning programme. This is a regulatory requirement because validated cleaning procedures contribute to the assurance of product purity and safety. This module provides a comprehensive account of equipment cleaning validation requirements and procedures. It follows the work of a pharmaceutical company's validation team as they establish and validate the cleaning program for a new production line.
VAL07: Computer Systems Validation, Part 2: Implementation
By Zenosis
This module describes the design, development and installation phase, the validation phase, and the operation and maintenance phase of the validation of computerised systems in medicines and healthcare products manufacturing environments. It continues to follow the progress of a pharmaceutical company's project to validate a new dispensary control system.
Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

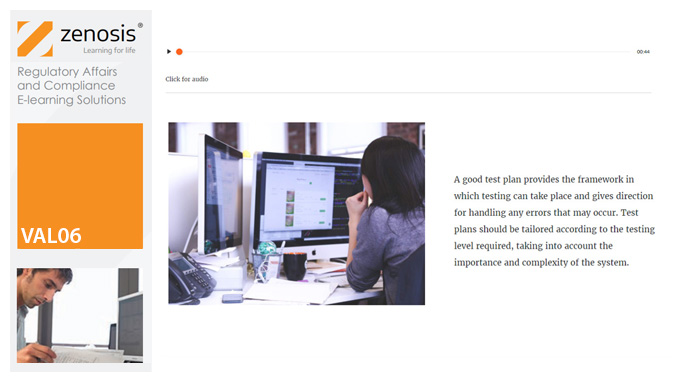
VAL06: Computer Systems Validation, Part 1: Planning
By Zenosis
In the medicines and healthcare products industries, computerised systems used in automated manufacturing or laboratory processes to which Good Manufacturing Practice requirements apply need to be validated. This module describes the planning of such validation. It follows the work of a pharmaceutical company's team as they validate the dispensary control system for a new production line.

Professional Certificate Course in Requirements Validation and Management in London 2024
4.9(261)By Metropolitan School of Business & Management UK
This Professional Certificate Course in Requirements Validation and Management offers a thorough exploration of the importance of validating achievable and realistic requirements. Participants will delve into diverse validation techniques, including reviews, inspections, and walkthroughs, while also gaining insights into monitoring, tracking, and effectively managing conflicting requirements to meet stakeholder expectations throughout the project lifecycle. After the successful completion of the course, you will be able to learn about the following: Understand the importance of validating requirements to ensure that they are achievable and realistic Discuss different techniques for validating requirements, such as reviews, inspections, and walkthroughs Understand the importance of monitoring and tracking requirements throughout the project lifecycle Explain how to handle conflicting requirements and manage stakeholder expectations This Professional Certificate Course in Requirements Validation and Management provides a thorough grasp of validating achievable requirements using diverse techniques like reviews and inspections. Participants also learn to monitor, track, and address conflicting requirements, ensuring stakeholder satisfaction throughout the project lifecycle. This Professional Certificate Course in Requirements Validation and Management imparts crucial skills, emphasizing the significance of ensuring achievable and realistic requirements through diverse validation techniques, alongside monitoring, tracking, and effective conflict resolution for enhanced stakeholder satisfaction. Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Project Requirements Validation and Management Self-paced pre-recorded learning content on this topic. Requirements Validation and Management Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course.The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience, Project Managers Business Analysts Requirements Engineers Software Developers Stakeholders in Project Development Quality Assurance Professionals Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

ChatGPT for Salesforce Development
By Packt
Learn to accelerate your Salesforce development with the power of ChatGPT. This course will show you how to use ChatGPT to write your own Apex triggers, batch Apex, test classes, validation rules, and flows, allowing you to streamline your development process and improve productivity.
