- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Electrical Power Failure Analysis and Investigations – Insightful Investigations For Precision Resolutions
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your expertise in electrical power failure analysis and investigations with EnergyEdge's insightful course for precision resolutions. Enroll now!

Good Clinical Practice Auditing - Principles and Practice
By Research Quality Association
Course Information Our comprehensive course is used as a gateway to those stepping into the world of auditing clinical studies. Tailored for those already acquainted with Good Clinical Practice (GCP) and those transitioning from other audit disciplines, this programme stands as a pivotal guide. Pre-existing knowledge of GCP will significantly enhance your learning experience in auditing against these guidelines. How is this course run? Engage in immersive workshops providing hands-on practice with auditing techniques in a GCP context. Our seasoned tutors, boasting extensive audit experience, intertwine theory with practical insights drawn from their own professional journeys. What will I learn? A comprehensive understanding of the historical backdrop and objectives driving Good Clinical Practice, incorporating the latest industry developments Solid grounding in quality assurance activities aligned with regulatory standards Insight into potential pitfalls within clinical trials and the pivotal role of auditors in addressing these issues Clarity on the roles and responsibilities inherent to clinical trials auditing Exposure to a diverse range of audit techniques complemented by illustrative examples and supportive documents A nuanced understanding of regulatory inspectors' activities Expanded professional networks to propel your auditing career forward. Benefits include: A clear understanding of the role of the auditor under Good Clinical Practice improved audits Improved Good Clinical Practice compliance for your clinical trials. This course is structured to encourage delegates to: Discuss and develop ideas Solve specific problems Examine particular aspects of Good Clinical Practice. Tutors Tutors will be comprised of (click the photos for biographies): Rosemarie Corrigan EVP Global Quality, Worldwide Clinical Trials Cathy Dove Director and Owner, Dove Quality Solutions Julie Kelly Associate Director, Clinical Quality Assurance, Corcept Therapeutics Susana Tavares Director of Research Quality Assurance, - Programme Please note timings may be subject to alteration. Day 1 12:30 Registration 13:00 Welcome and Objectives for the first day of the course 13:30 Laying the Foundations Introduction to the clinical development process, the concepts of quality assurance, quality control and audit. 14:30 Break 15:00 Patient Protection Requirements for informed consent and ethics committee. Access to source documentation. Including a patient protection exercise. 16:05 Workshop 1 - Case Study on Informed Consent 16:45 End of Day Questions and Answers 17:00 Close of Day Day 2 08:50 Questions and Answers from Day 1 09:00 Effective Site Audits The procedures involved in selecting and setting up audits at investigator sites. 09:40 Workshop 1 - Planning the Effective Audit 10:30 Break 10:45 Source Data Verification The need for and purpose of verifying data. 11:25 Workshop 2 - Source Data Verification 12:30 Lunch 13:30 IMP Management The requirements surrounding the distribution of investigational medicinal products. Accountability from release to destruction. 14:15 Critical Document Audits The conduct of other study specific audits including protocols, databases and reports. 15:00 Break 15:15 Non-compliance Determining the acceptability of data. 16:00 Fraud - Fact or Fiction? How to identify fraud and its consequences 16:45 End of Day Questions and Answers 17:00 Close of Day Day 3 08:50 Questions and Answers from Days 1 and 2 09:00 Auditing Third Parties A review of audits of contract research organisations. 10:00 System Audits The concept of auditing processes across many clinical trials, including a practical exercise in process mapping. 10:45 Break 11:00 Workshop 3 - Process Mapping 11:45 Effective Audits Where theory meets reality. 12:30 Lunch 13:20 Audit Reports - Closing the Loop An examination of the processes which follow the evidence gathering phase of the audit. 14:20 Workshop 4 - Audit Reports Audit reports, corrective and preventive action. 15:00 Break 15:10 Regulatory Inspection Auditors and regulatory inspections -how the QA team can help the organisation to perform during a regulatory inspection. 15:55 Final Questions and Answers 16:10 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 17 Points Development Level Develop

An Understanding of âSuprapubic Catheter Care
By Guardian Angels Training
Gain a comprehensive understanding of suprapubic catheters with our course. Learn about indications, insertion procedures, and ongoing care to ensure optimal outcomes for patients.
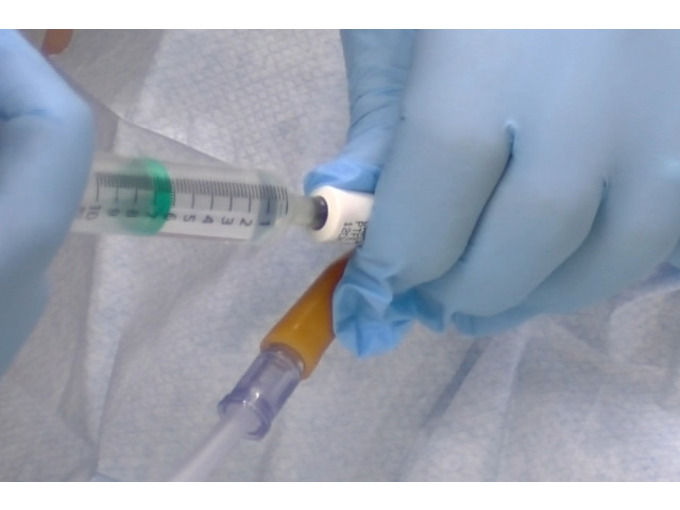
Biohazard Safety: Managing Blood and Body Fluid Spillages Instructor
By Guardian Angels Training
Gain expertise in biohazard safety with our "Biohazard Safety: Managing Blood and Body Fluid Spillages Instructor Training" course. Ideal for healthcare professionals, lab staff, and emergency responders.
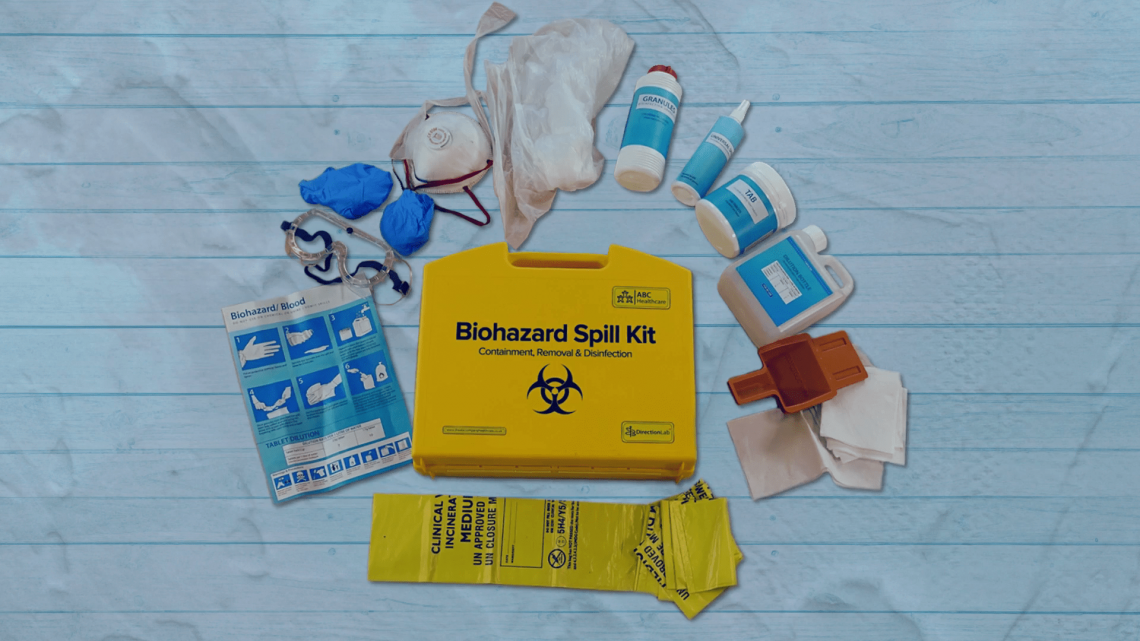
The Auditing Course
By Research Quality Association
Course Information Designed to develop personal proficiency in audit planning, execution and reporting, this course is meticulously crafted to refine essential audit skill sets. Through immersive scenarios focused on on-site audit conduct (with an alternative Remote Auditing Course available), participants will engage deeply in the audit process. Extending Expertise: Applicable across all audit types, this course builds upon and enriches the foundational concepts taught in RQA's suite of research quality assurance courses. From 'Research Quality Assurance for Good Laboratory Practice' to 'Good Clinical Practice Auditing – Principles and Practice' and 'Good Manufacturing Practice for Investigational Medicinal Products,' this programme extends the scope of learning. Relevance and Value: Relevant to any area of regulated research and development, this course shines particularly in contexts mandating a quality system for audit. Participants with prior audit experience will gain maximum value from this course. Key Benefits: Enrich your skill set to: Navigate audit processes encompassing planning, execution, reporting, and follow-up Embrace a personalised approach fostering positive audit outcomes Analyse evidence and present cohesive audit findings Recognise the pivotal role of audits in driving continual improvement. Interactive Learning: Structured to foster dynamic engagement, this course encourages delegates to: Engage in discussions, idea development, and problem-solving Exchange invaluable information and experiences. Hands-On Experience: A highlight of this course is the series of practical workshops, where delegates work in small syndicate groups, applying the acquired skills from lectures into real-world scenarios. Tutors Tutors will be comprised of (click the photos for biographies): Andrew Waddell Founder Director, Tower Mains Ltd Rosemary Ichaba Senior QA Associate, Tower Mains Ltd Cate Ovington Director, The Knowlogy Group Ltd Jean McWilliam Associate Director, Alexion View pop up Programme Please note timings may be subject to alteration. Day 1 08:45 Registration 09:00 Welcome and Course Objectives 09:10 What is 'Audit'? Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 09:30 Audits and their Purpose The concepts of quality assurance, quality control, quality management and audit are discussed. 10:30 Break 10:45 Audit Planning The requirements for an effective audit programme and individual audit plans. 11:30 Workshop 1 - Getting the Audit Started Planning for the audit. 12:25 Workshop 1 - Feedback 12:45 Lunch 13:30 Workshop 2 - Getting the Audit Started Arranging the opening meeting. 13:50 Workshop 2 - Feedback Audit initiation. Review and discussion of the role of the opening meeting. 14:25 Auditing Techniques (1) - Data and Documentation Techniques for the conduct of data and report audits are investigated. 14:55 Break 15:10 Workshop 3 - Data and Documentation Audit Conducting an audit of a data package and supporting documentation. 17:15 Close of Day Day 2 09:00 Auditing Techniques (2) - The People Questioning techniques which get the required information from the auditee. 09:45 Live Audit Role Play Auditor and auditee behaviours are explored and strategies developed for successful audit interactions. 10:15 Break 10:35 Audit Closing Meeting An exploration of audit closing meetings. 11:00 Workshop 4 - Audit Observations and Preparing for the Closing Meeting Reviewing and categorising your observations and getting ready to present your case. 11:45 Workshop 4 - Feedback 12:30 Audit Reports The content and distribution of an effective audit report are investigated and the importance of effective written communication is discussed. 13:00 Lunch 13:45 Workshop 5 - Audit Reports and Follow-up Mechanisms for promoting effective corrective and preventive action. Critical review of an audit report example. 14:30 Workshop 5 - Feedback 14:55 Corrective and Preventive Action and Follow-up The auditor's role in monitoring responses to audit and the corrective and preventive actions promised is explored. 15:20 Panel Session An opportunity to get answers to outstanding questions. 15:30 Close of Course Extra Information Course material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. CPD Points 14 Points Development Level Develop

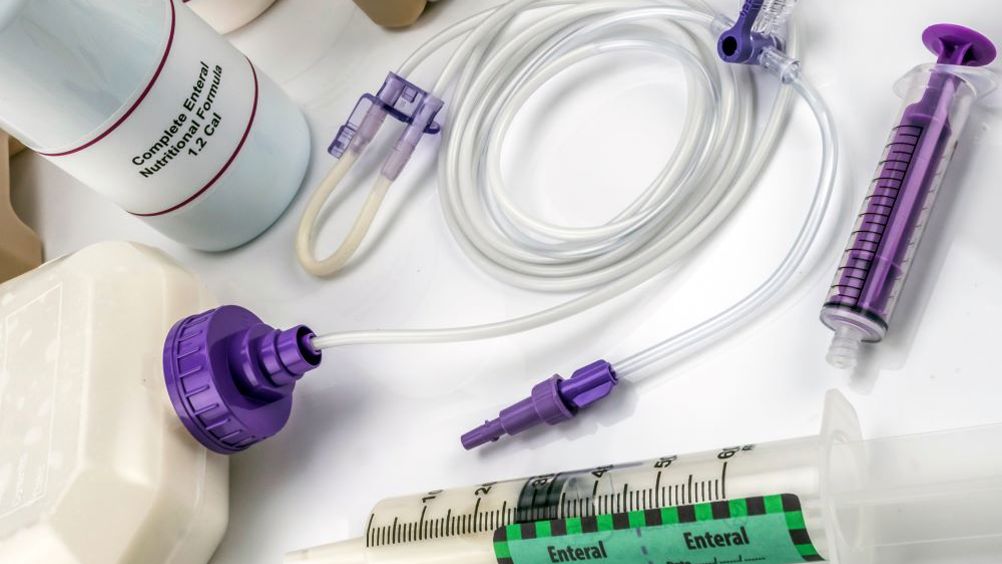
An Understanding of Enteral Tube Care and Feeding
By Guardian Angels Training
Gain comprehensive knowledge and practical skills in enteral tube care and feeding with our course. Learn about insertion techniques, maintenance, feeding methods, and addressing complications to ensure safe and effective patient care.
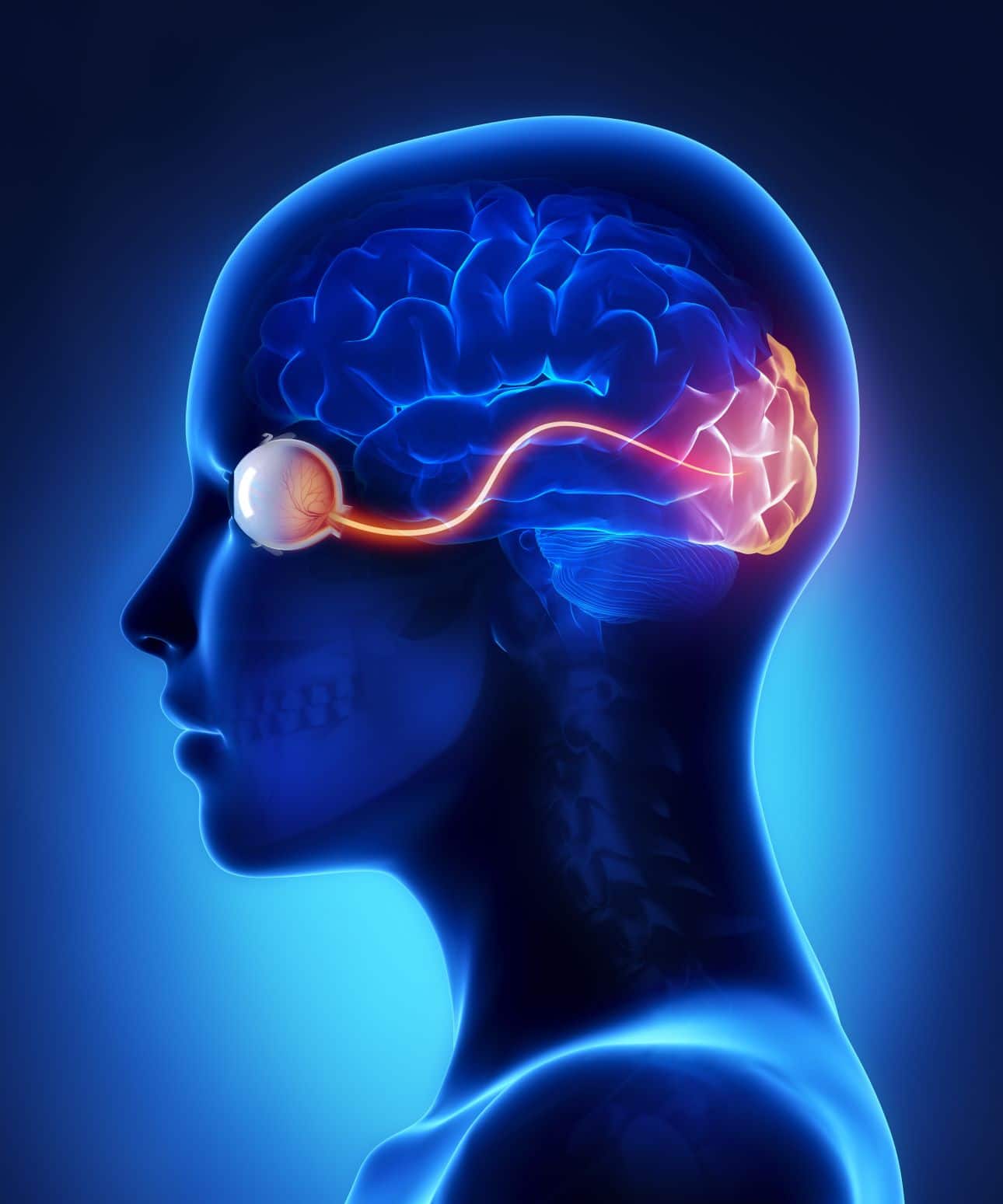
An Understanding of Physiological Observations
By Guardian Angels Training
Gain essential knowledge and practical skills to accurately monitor and interpret physiological parameters in patients with our "An Understanding of Physiological Observations" course. Learn to conduct and interpret various observations to support informed clinical decision-making.
An Understanding of Nasogastric Tube (NGT) Insertion and Feeding Training
By Guardian Angels Training
Nasogastric tube training ensures healthcare professionals have the correct clinical knowledge and skills to provide excellent care and support for individuals who may require treatment via NG tube for nutrition, hydration or medication.

Understanding Preventable Diseases for HCAs
By M&K Update Ltd
This course provides the underpinning physiology and disease process knowledge for HCAs, working with patients with preventable diseases.

Work Breakdown Structures
By IIL Europe Ltd
Work Breakdown Structures It's amazing how often project managers begin the project planning process by making an outlined list of every task they believe will be required to complete a project and then proclaim they have created the work breakdown structure (WBS) for the project. The result is a list of hundreds, or even thousands of tasks, many of them having durations of a few days or a few hours. Essentially, what they have done is create a 'to do' list, which they then use as a 'checklist' to measure progress. This approach leads to, and even encourages, micromanagement of the resources working on the project without consideration of more critical aspects of project management such as: requirements management, risk management, procurement management, estimating, scheduling, executing, and controlling. Further, it makes it impossible to see the big picture, at levels of detail, in keeping with the needs of sponsors, clients, project and functional managers, team leaders, and project performers. Join us for this exciting program and learn how to use the WBS to make better-informed business decisions. What You Will Learn You will learn how to: Describe the need for a project WBS Describe the WBS role in the project Gain practical experience in the development, decomposition, and use of the WBS Determine the appropriate level of detail in the WBS. Explain how the WBS integrates with project requirements, risk, procurement, estimating, scheduling, and overall project execution. Provide the basic tools to enhance efficient re-use of key information in your future projects Foundation Concepts Key definitions History of the WBS Importance of the WBS Overall structure Terminology Other breakdown structures WBS tools WBS & Scope Project scope management processes Specification of the project objectives WBS design based on project deliverable WBS decomposition process and 'The 100% rule' Work Packages and Control Accounts WBS & Risk Risk management planning and WBS Risk identification to enhance the WBS Risk analysis and the WBS Risk responses and updating the WBS Implementing risk response and Monitoring risks and the WBS WBS & Estimating Use of WBS in the estimating process Components and work packages Sizing and algorithmic estimates WBS & Scheduling Component Scheduling - High-Level Milestones WBS activity decomposition WBS elements dependencies Work Package Level Schedules Responsibility assignment matrix WBS & Execution and Control Earned Value Management and tracking of work performance Progress reports, forecasts, and corrective and preventive actions used to manage work performance Necessary information to close out a project

Search By Location
- Prevent Courses in London
- Prevent Courses in Birmingham
- Prevent Courses in Glasgow
- Prevent Courses in Liverpool
- Prevent Courses in Bristol
- Prevent Courses in Manchester
- Prevent Courses in Sheffield
- Prevent Courses in Leeds
- Prevent Courses in Edinburgh
- Prevent Courses in Leicester
- Prevent Courses in Coventry
- Prevent Courses in Bradford
- Prevent Courses in Cardiff
- Prevent Courses in Belfast
- Prevent Courses in Nottingham