- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
City & Guilds F-Gas 2079 Category 1
By Rachp School
About this course RACHP School offer 4 days training with day 5 for theory and practical examination in our F-Gas 2079 Cat 1. Morning sessions are spent learning theory, while afternoon sessions are spent carrying out practical tasks such as recovering refrigerant from systems, removing a section, carrying out a braze with flared connections, pressure testing, vacuuming system, recharging and recording on services sheets (including sub-cooling and superheat measurements). This is a safe handling of refrigerants course so correct purge process will be taught. The theory exam is a closed book 80-minute online exam which requires a 60% (24 correct answers) pass mark. This qualification allows engineers to legally work on refrigeration systems under the EC 842/2006 F-Gas regulations. What is covered on this course? Expert instruction – Learn from experienced instructors with deep knowledge of F-Gas 2079/11 & ODS regulations. Hands-on training: Gain practical skills through real-world scenarios. The Practical elements will include: Correct PPE and understanding of the Health & Safety at Work act. Correct isolation procedures of a system before carrying out any works. Safe handling of refrigerants, including correct procedure to purge hose lines correctly. Removing a section of pipe, cutting, expanding and flaring a new piece of pipe with a braze that will be pressure tested to PED regulations. Vacuuming the system, recharging and recording on services sheets subcooling and superheat measurements, including air on and air off and working out the delta T in the Kelvin scale. Understand the pressure/temperature relationship. Working out the compressor ratio and evaluating if the system is running within design parameters, and if not, what can be done to improve efficiency. Correct waste disposal procedures of waste oil/refrigerant. A “Peel Test” of a brazed joint that the student will carry out. There are 2 exams, one theory and one practical both held on the Friday.

3ds Max Character Animation Training Course
By ATL Autocad Training London
Who is this course for? 3ds Max Character Animation Training Course. Master character animation in our 3ds Max course. All levels welcome. Learn from certified tutors in flexible in-person or online sessions. Create unique 3D characters from scratch, gaining personalized techniques to fuel your creativity. Click here for more info: Website Duration: 20 hours Method: 1-on-1 personalized attention Schedule: Flexible 1-on-1 sessions. Schedule your sessions at your convenience, choosing any hour between 9 am and 7 pm from Mon to Sat. Course Title: 3ds Max Character Animation Workshop Duration: 20 Hours Course Overview: This workshop is meticulously crafted to instill the foundational principles of character animation utilizing 3ds Max. Whether you're a novice or possess some background in 3D modeling and animation, this course caters to your learning needs. You'll delve into the art of character rigging, grasp animation essentials, and employ advanced methods to breathe life into your characters. Course Outline: Module 1: Introduction to Character Animation Grasping animation principles Exploring 3ds Max animation tools Mastering character rig creation Understanding the intricacies of the timeline Module 2: Basic Animation Principles Embracing keyframe dynamics Crafting fundamental animation cycles Applying the 12 principles of animation Navigating the graph editor Utilizing ease-in and ease-out techniques Module 3: Advanced Animation Techniques Harnessing the power of the reaction manager Crafting non-linear animations Implementing inverse kinematics for dynamic movements Designing custom controllers Exploring expressions and scripts Module 4: Character Creation Sculpting a character model from scratch Grasping the nuances of topology Perfecting UV maps and texturing techniques Preparing characters for seamless rigging Module 5: Facial Animation Mastering facial animation principles Creating expressive blend shapes Utilizing morph targets for nuanced expressions Achieving flawless lip syncing Module 6: Body Animation Crafting seamless walk cycles Animating characters in motion Creating authentic and believable poses Employing character physics for lifelike movements Module 7: Advanced Character Animation Implementing motion capture data for realistic animations Leveraging CAT and Biped for intricate movements Understanding motion blur nuances Adding special effects for enhanced realism Fine-tuning rendering and outputting animations Module 8: Character Animation Projects Synthesizing knowledge into practical applications Creating a fundamental character animation Crafting a nuanced walk cycle Executing complex character animations Course Requirements: Access to a computer with 3ds Max installed Basic proficiency in computer operations Enthusiasm for delving into the world of character animation Course Goals: Upon completion, you will possess a profound understanding of character animation in 3ds Max. You'll be equipped with the expertise to create intricate, lifelike character animations using advanced techniques. Moreover, you'll gain the skills necessary to continue honing your craft, ensuring a solid foundation for your future endeavors in the realm of character animation. Upon successful completion of the 3ds Max Character Animation Workshop, participants will: Master Fundamental Principles: Understand the core principles of character animation, including keyframe dynamics, timing, and the 12 principles of animation, laying a strong foundation for advanced techniques. Proficient Software Usage: Navigate 3ds Max confidently, utilizing animation tools, character rigging techniques, and specialized editors for precise control over character movements. Advanced Animation Techniques: Apply advanced techniques such as non-linear animations, inverse kinematics, and custom controller design to create dynamic and realistic character movements. Facial Animation Mastery: Demonstrate expertise in facial animation by creating expressive blend shapes, morph targets, and achieving seamless lip syncing for realistic character emotions. Body Language Proficiency: Create fluid and natural body movements, including walk cycles, dynamic poses, and character motions, capturing the essence of lifelike animations. Special Effects Integration: Integrate special effects seamlessly into character animations, enhancing visual appeal and realism in the final output. Project Implementation: Apply acquired knowledge and skills in practical projects, including basic character animations, walk cycles, and complex character animations, demonstrating proficiency in real-world scenarios. Problem-Solving Skills: Develop problem-solving abilities related to character animation challenges, employing creative solutions to achieve desired results. Collaborative Skills: Engage in collaborative projects, demonstrating effective communication and teamwork while integrating animations into broader creative contexts. Portfolio Enhancement: Build a robust portfolio showcasing diverse character animations, reflecting both technical prowess and creative expression, essential for career advancement in the animation industry. Continued Learning: Acquire the skills and confidence necessary to pursue further learning and self-improvement in the field of character animation, enabling a continuous growth trajectory in the industry. Course Title: 3ds Max Character Animation Workshop Duration: 20 Hours Key Details: Course Focus: Comprehensive training in character animation using 3ds Max, covering fundamental principles, advanced techniques, facial animation, body language, special effects integration, and project-based learning. Audience: Ideal for beginners and individuals with some background in 3D modeling and animation, aiming to enhance their skills in character animation for industries such as animation studios, gaming, and film production. Instruction Method: Interactive, instructor-led sessions combining theoretical knowledge with hands-on practical exercises, fostering a dynamic learning environment. Flexible Learning Options: Participants can choose between in-person and live online sessions, accommodating diverse schedules and geographical locations. Certified Instructors: Experienced tutors and industry professionals with certification in 3ds Max and character animation, ensuring high-quality instruction and personalized guidance. Project-Based Learning: Engage in real-world projects, applying learned skills to create character animations, walk cycles, and intricate character movements, fostering practical expertise. Software Proficiency: Gain proficiency in 3ds Max, including animation tools, character rigging, and specialized editors, enabling participants to confidently navigate the software. Collaborative Learning: Opportunities for teamwork and collaborative projects, encouraging effective communication and networking within the class. Career Development: Build a diverse and impressive portfolio, receive guidance on industry best practices, and develop problem-solving skills crucial for a successful career in character animation. Post-Course Support: Access to resources, tutorials, and community forums, allowing participants to continue learning and stay updated with industry trends even after the course completion. Certification: Participants receive a certificate of completion, recognizing their proficiency in 3ds Max character animation, enhancing their professional credibility in the job market. By enrolling in this course, you'll enjoy the following advantages: Comprehensive Learning: Master the art of character animation in 3ds Max, covering fundamental concepts and advanced techniques. Certified Tutors and Industry Experts: Learn from experienced professionals with extensive knowledge of character animation, providing valuable insights. Personalized Instruction: Receive one-to-one training tailored to your specific learning needs, ensuring individual attention and effective progress. Flexible Learning Options: Choose between in-person or live online training, offering convenience and accessibility to suit your schedule. Recorded Lessons: Access recorded sessions to review content and reinforce your learning at your own pace and convenience. Lifetime Email Support: Benefit from ongoing assistance and guidance through email, even after completing the course. Free Career Advice: Tap into our industry expertise and receive valuable career guidance to excel in the field of character animation.

Sacred Soul Healing Odyssey in London "A Journey of Sound, Spirit, and Soul Awakening."
4.8(6)By Steph Edwards
A Journey of Sound, Spirit, and Soul Renewal. The Sacred Soul Healing Odyssey one day retreat in London is a transformative journey of deep healing, feeling your true nature, and inner restoration and embodiment. Experience deep healing and rediscover your true essence. Immerse yourself in a powerful journey of inner restoration and embodiment. Don’t miss this opportunity to transform your self! Key Elements: Vibroacoustic Therapy: Participants will be enveloped in the soothing vibrations of low-frequency sound waves, allowing the body to enter a state of profound relaxation and stress reduction. Hands-On Healing: To enhance the body's natural healing processes and promote a sense of grounding, balance and integration. Trauma Release Exercises: Through various exercises and gentle shaking, participants will start to release stored emotional and physical tension in the hips and body, encouraging a deeper sense of liberation and self-awareness. Plant Medicine Ceremony: The integration of carefully selected plant medicines (Sacred Cacao paired with the high vibrational Sacred Awareness Flower Essence by Lotuswei) will provide an additional layer of holistic support, spiritual connections and breakthroughs. Meditation and Mindfulness: Participants will engage in a variety of meditations, including Yoga Nidra and visualisation, designed to foster inner calm, clarity, and a deeper connection to the present moment. Breathwork: Participants will be guided through the breath allowing more of life's energy to flow into the body, enabling deep healing and mental clarity. Who Should Attend: This retreat is designed for individuals seeking presence and insight into their spiritual self, clearing out stuck energy and feeling their true nature. About Eduardo Camargo - Eduardo embraces ancestral and alternative healing methods, including holotropic breathwork, sound healing, and Amazonian plant medicine. Spiritual well-being became his core focus, leading Eduardo to practice the Wim Hof method. Through breathwork, vibration, and nature’s medicines, Eduardo first healed himself and is now helping others on their journey. Eduardo integrates ancient wisdom with modern life, through spiritual healing and personal growth to provide a profound experience. About Steph Edwards - Steph is an Intuitive Bodyworker, blending CranioSacral Therapy, Reiki, Reflexology, and Energy Healing to craft personalised sessions. Her intuitive approach allows her to connect with your unique needs in the present moment, merging the art of bodywork with energetic healing. Through a profound spiritual practice with Sacred Cacao and plant medicines, Steph cultivates sacred spaces that can catalyse self-discovery and genuine connection back to your true nature. Sneak peek

Master Business Networking in Just 1 Day - Join our Workshop in Heathrow
By Mangates
Business Networking 1 Day Training in Heathrow

Venepuncture and cannulation course Venepuncture training for healthcare professionals Cannulation skills development Intravenous access techniques Blood sample collection training Infection control in venepuncture Hands-on venepuncture practice Cannula insertion training Nursing revalidation hours CPD accredited course Healthcare professional skills development Venepuncture and cannulation procedures Venepuncture certification program Intravenous catheter insertion Best practices in venepuncture Patient assessment for venepuncture Troubleshooting venepuncture complications Venepuncture and cannulation simulation Real-life venepuncture scenarios Healthcare career advancement with venepuncture skills
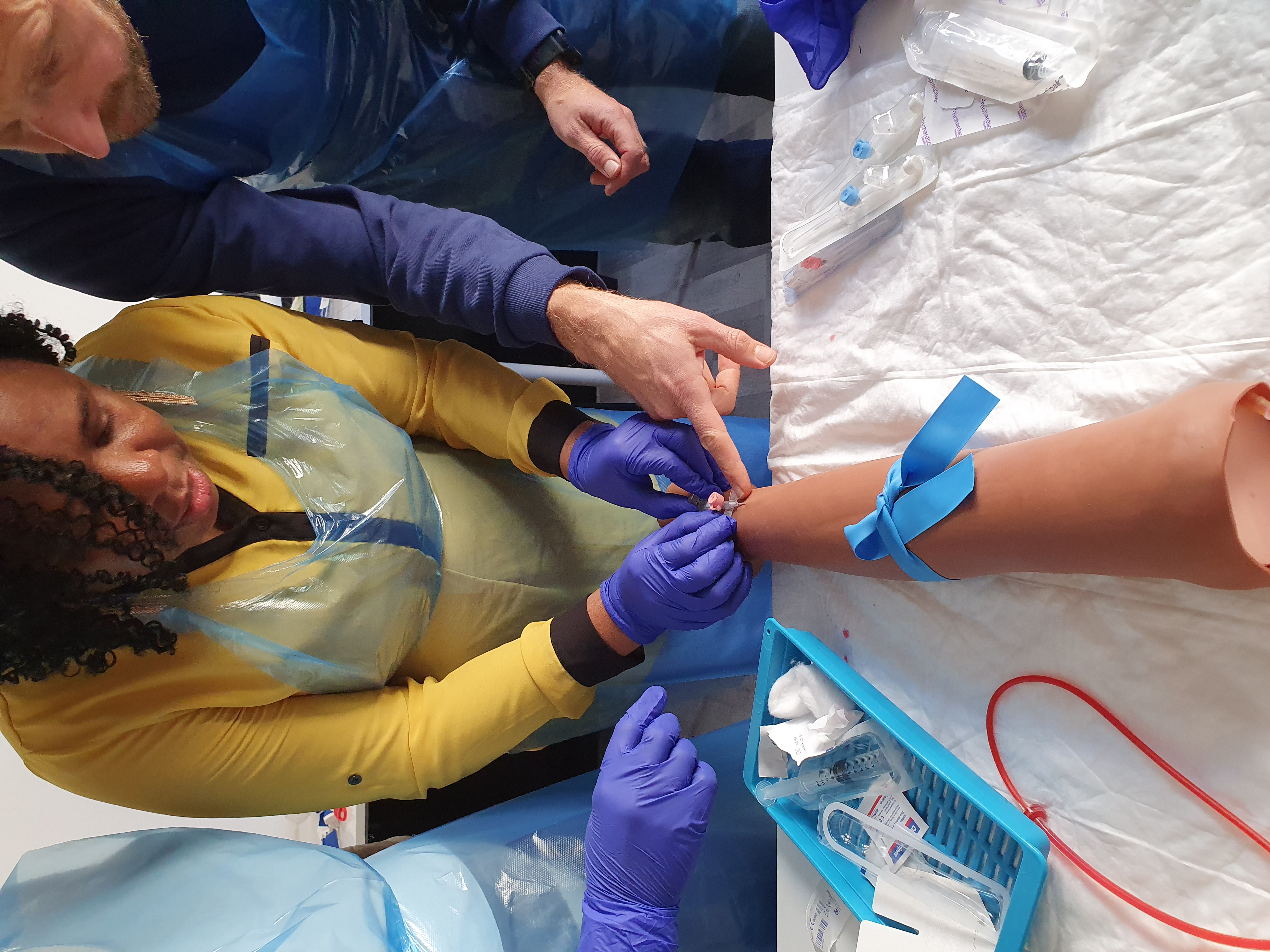
, The Intravenous Route, Bioavailability, the First Pass Effect, IV drug administration Vascular Access Devices Care & Management: Peripheral Cannula, Midline, Catheter, different Central Venous Access(care of Hickman line), PICC, Implantable Port, UVC and subcutaneous infusion, VAD Assessment, Risk & Complication of IV Therapy, Infection control, Allergy, Fluids & Electrolytes and Drug Calculations. Total Parenteral Nutrition –TP, Solution content, Administration, Routes for delivery,
NPORS Lift Supervisor Training The aim of the NPORS Lift Supervisor Training is to Provide candidates with underpinning knowledge to allow them to understand the role and responsibility of the Lift Supervisor. As a result of the Lift Supervisor Course, and following successful completion of the NPORS Crane Supervisor training candidates will be able to understand and follow safe systems of work for lifting operations. This Lift supervisor course is for 3 days and can be completed at your site or ours. It is important that all delegates have a good understanding of spoken and written English for NPORS Crane Supervisor Training. NPORS Lift Supervisor Experienced Test Book with Confidence at Vally Plant Training At Vally Plant Training, we guarantee unbeatable value with our Lift Supervisor Experienced Test Price Match Promise. When you choose us, you can book with confidence, knowing that we will not be beaten on price. If you find a lower price for the same NPORS Lift Supervisor Experienced Worker Test, we’ll match it—ensuring you receive top-quality training at the best possible rate. Click for our terms and conditions Your skills, our commitment—always at the best price. NPORS Lift Supervisor Experienced Worker Test is for operators who have received some form of Lift Supervisor Course in the past or alternatively has been working with Lifting equipment, like cranes, Excavators or Telehandlers for a number of years. If you are unsure if you qualify to go down the Lift Supervisor experienced test route please contact our team to discuss this in more detail. Discounts are available for multiple Lift Supervisor Course bookings There are two parts to the lift supervisor test, a theory section comprised of 25 questions and a practical session, however Lift Supervisor training revision notes will be sent once the test has been booked. It is important that all delegates have a good understanding of spoken and written English for NPORS Crane Supervisor Training Crane Supervisor Course Summary: Leading Safe and Efficient Lifting Operations Introduction Ever wonder who keeps construction sites and warehouses running smoothly and safely? That’s where lift supervisors come in. They’re the unsung heroes ensuring everything moves like clockwork. And when it comes to proving you’re the best in the biz, NPORS certification is your golden ticket. It’s not just a piece of paper; it’s your passport to climbing the career ladder. Choose our Lifting Supervisor Course Today. Why Choose Our NPORS Lift Supervisor Training? What makes our training stand out with our Lifting Supervisor Course? Imagine learning from folks who’ve been in the trenches, in training grounds that feel like the real deal, and schedules that bend to your life, not the other way around. We’re not about boring lectures; we’re about getting your hands dirty. Who Should Attend Lift Supervisor Training? Are you the go-to person when things need to get done? Whether you’re starting out or looking to step up, if you’re in the world of construction or logistics, this Lifting Supervisor Course is for you. It’s tailored for those who like to keep things moving, safely and efficiently. Course Objectives: 1. Understanding Regulatory Requirements: Familiarise participants with relevant regulations and industry standards governing crane operations, LOLER. Ensure compliance with legal requirements and best practices for safe lifting operations, BS7121. 2. Roles and Responsibilities of a Crane Lift Supervisor: Define the roles and responsibilities of a Lift supervisor within the context of lifting operations. Highlight the importance of effective communication, leadership, and decision-making skills. 3. Crane Safety Procedures: Provide an overview of crane safety procedures, including pre-operational checks, equipment inspection, and maintenance. Emphasize the importance of hazard identification, risk assessment, and mitigation strategies. 4. Lifting Plan Development: Guide participants in the understanding of the lifting plans tailored to specific lifting tasks and site conditions created by the Appointed Person(AP). Address factors such as load weight, size, shape, centre of gravity, and environmental considerations. 5. Site Safety and Hazard Awareness: Enhance participants’ awareness of potential hazards in the lifting environment, such as overhead power lines, unstable ground, and confined spaces. Implement effective measures to mitigate risks and ensure a safe working environment. 6. Communication and Coordination: Stress the importance of clear and effective communication between crane operators, riggers, signallers, and other personnel involved in lifting operations. Provide guidance on establishing communication protocols, using standardized hand signals, and conducting pre-lift briefings. 7. Emergency Response and Crisis Management: Equip participants with the skills and knowledge to respond effectively to emergencies and crisis situations during lifting operations. Implement emergency procedures, evacuation protocols, and contingency plans to mitigate risks and ensure personnel safety. 8. Practical Exercises and Case Studies: Provide hands-on lift supervisor training opportunities for participants to apply theoretical knowledge in practical scenarios. Analyse real-life case studies to identify lessons learned, best practices, and areas for improvement in crane supervision. Learning Outcomes By the end, you’ll be a pro at keeping sites safe, managing lifts, and leading teams. You’ll walk away not just with knowledge, but with practical skills that meet and beat industry standards. It’s about making you the go to lift supervisor everyone wants on their team. Course Logistics Ready to jump in? We’ve got training spots across the UK, with dates and times that fit your life. Signing up is a breeze, and we’ll guide you through any paperwork or prerequisites. It’s all about making it easy for you to get started. Conclusion: A crane supervisor course aims to empower participants with the expertise and confidence to lead safe and efficient lifting operations on construction sites. By focusing on regulatory compliance, safety procedures, lifting plan development, hazard awareness, communication, and practical training, the course prepares crane supervisors to fulfil their roles effectively and ensure the well-being of all personnel involved in lifting activities. Investing in crane supervisor training is essential for promoting a culture of safety, minimising risks, and achieving excellence in crane operations management. Crane Supervisor Training Available 7 days a week to suit your business requirements. VPT have a team of friendly and approachable instructors, who importantly have a wealth of knowledge of lifting supervision and the construction industry We have our own training centre conveniently located close to the M5 junction 9, In Tewkesbury. With its own purpose-built practical training area to simulate an actual working environment for the supervisor course. Our Lift Supervisor training and test packages are priced to be competitive. Discounts are available for multiple bookings We can send a fully qualified NPORS supervisor Tester to your site nationwide, for instance to reduce the amount of time away from work More courses: Polish your abilities with our dedicated Lift Supervision Training, Slinger Signaller Training, Telehandler Training, Cat & Genny Training, Plant Loader Securer, Ride-On Road Roller, Abrasive Wheel Training, Lorry Loader Training and Scissor Lift Training sessions. Learn the safe and effective operation of these vital machines, crucial for construction and maintenance tasks. Elevate your skills and career prospects by enrolling in our comprehensive courses today. Frequently Asked Questions 1. What is Lift Supervisor Training? Lift Supervisor Training is a specialised course designed to equip individuals with the knowledge and skills required to supervise lifting operations safely and efficiently. This training typically covers topics such as planning lifts, managing lifting equipment, and ensuring compliance with safety regulations. 2. Who should attend The Lifting Supervisor Course? This training is ideal for individuals responsible for overseeing lifting operations on construction sites, in warehouses, or any environment where lifting equipment like cranes are used. It’s particularly beneficial for site supervisors, managers, and anyone involved in the planning and execution of lifting operations to attend the NPORS Lift supervisor Course. 3. What certifications are available through the Lift Supervisor Training? Participants can obtain several Lift Supervisor certifications, including: NPORS Traditional card: Valid for 5 years, widely accepted in various sectors. NPORS card with CSCS logo: Recognised by major building contractors, with an initial RED trained operator card that can be upgraded to a BLUE competent operator card after completing relevant Crane Supervisor NVQ. 4. Is a health and safety test required for the NPORS Crane Supervisor Red operator card with the CSCS logo? Yes, to qualify for this card, you must have completed the CSCS operatives health and safety test within the last two years. 5 . How long is the certification valid, and what is the renewal process? The NPORS Traditional card is valid for 5 years. The NPORS card with the CSCS logo’s RED trained operator card is valid for 2 years, after which it can be upgraded to a BLUE competent Crane Supervisor operator card upon completion of relevant NVQs. The renewal process typically involves undergoing a refresher course or assessment to ensure continued competence. For those looking for a “NPORS Crane Supervisor Training near me,” our widespread operations make it convenient for you to access Vally Plant Trainings top-quality training no matter where you are in the UK

Search By Location
- Cat Courses in London
- Cat Courses in Birmingham
- Cat Courses in Glasgow
- Cat Courses in Liverpool
- Cat Courses in Bristol
- Cat Courses in Manchester
- Cat Courses in Sheffield
- Cat Courses in Leeds
- Cat Courses in Edinburgh
- Cat Courses in Leicester
- Cat Courses in Coventry
- Cat Courses in Bradford
- Cat Courses in Cardiff
- Cat Courses in Belfast
- Cat Courses in Nottingham
