- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Greenhouse Gas Accounting, Mitigation, Certification and Reporting
By EnergyEdge - Training for a Sustainable Energy Future
Join EnergyEdge's course to gain valuable insights into greenhouse gas accounting, mitigation, certification, and reporting.

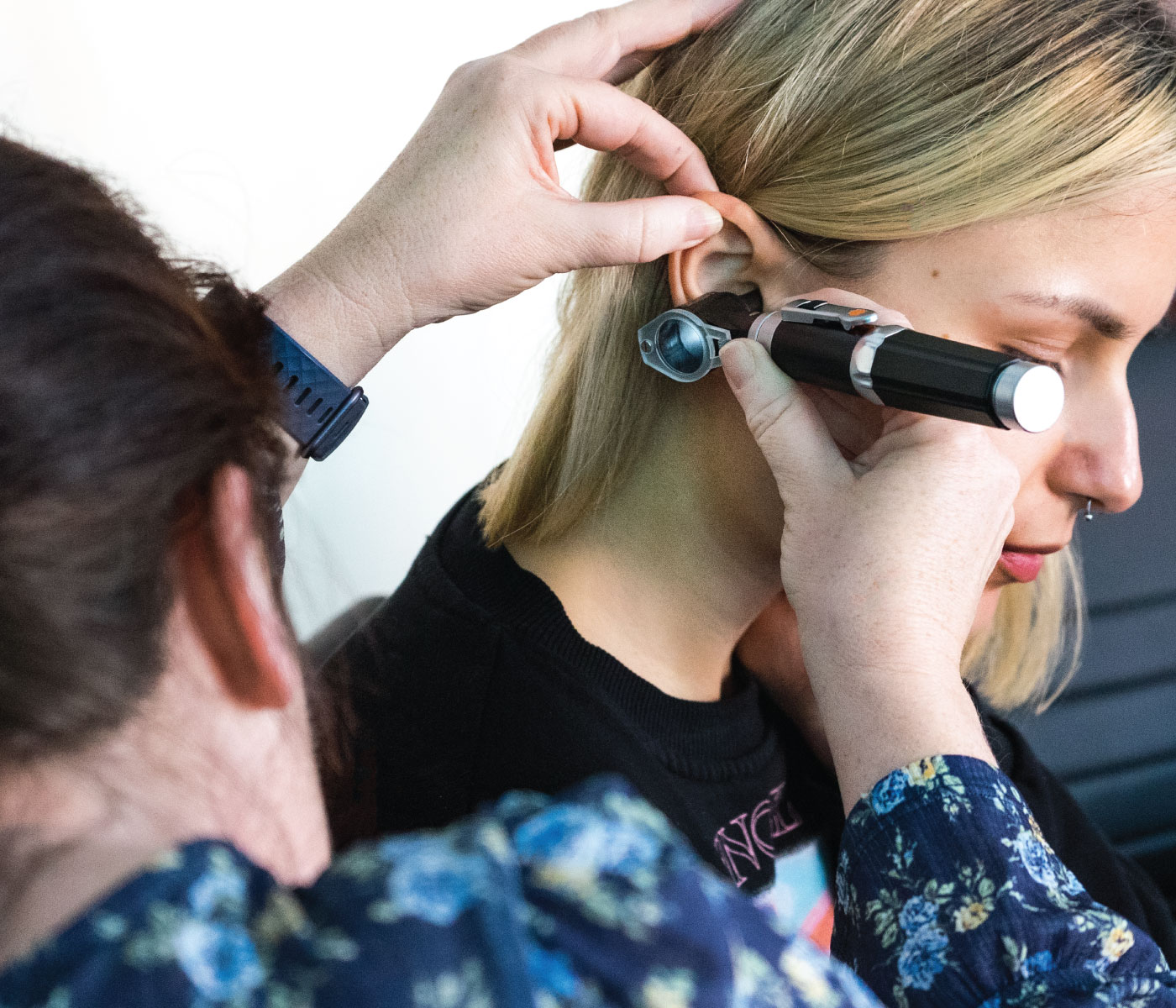
An Understanding of Ear Assessment and Care Update (including Ear Irrigation)
By Guardian Angels Training
Enhance your ear assessment and care skills with our comprehensive course on ear irrigation. Gain practical knowledge and updated insights for optimal ear hygiene.
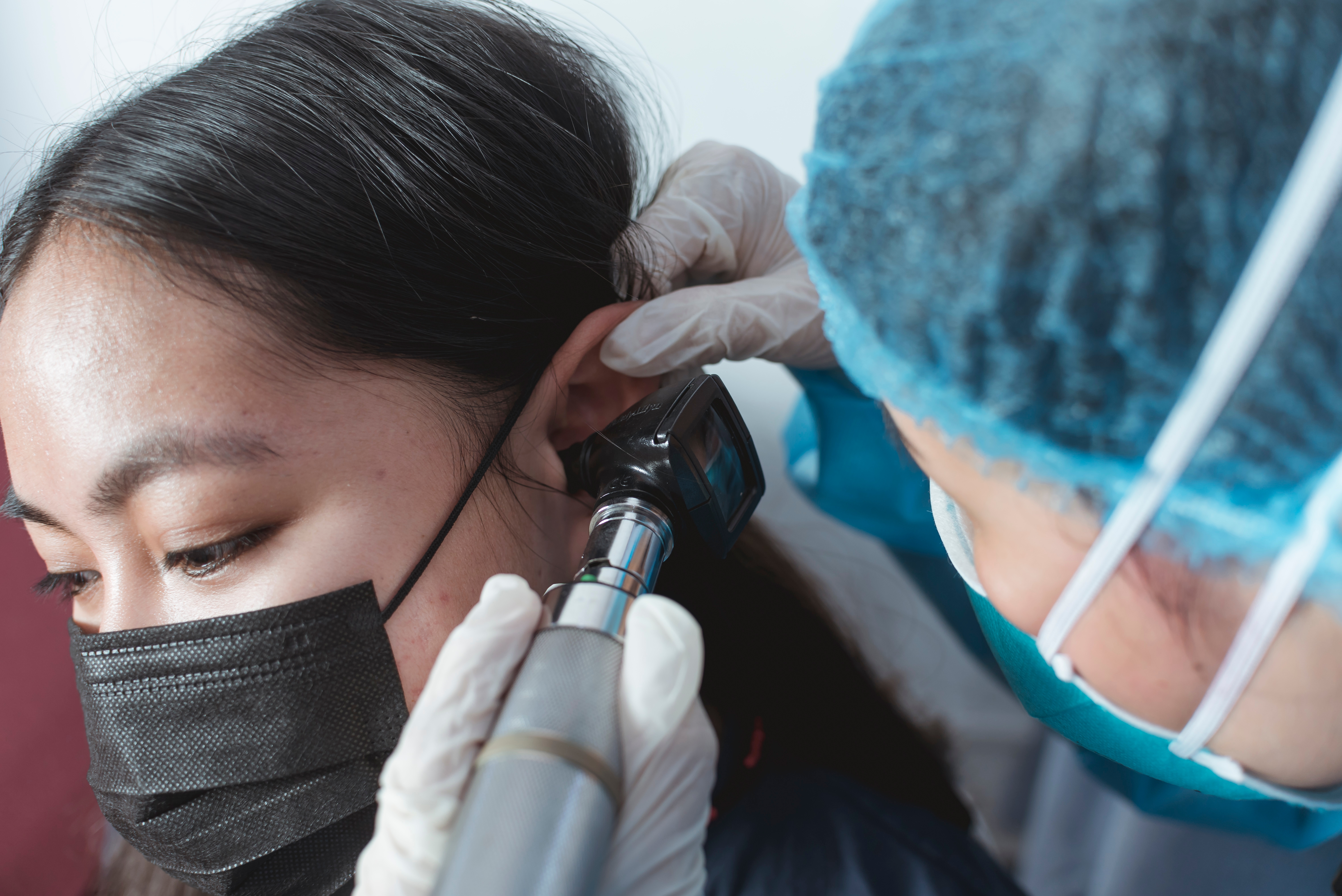
An Understanding of Ear Anatomy and Otoscopy
By Guardian Angels Training
Gain comprehensive knowledge of ear anatomy and otoscopy with our course. Ideal for healthcare professionals, medical students, and audiologists.
The BrainChild Foundation Course: Identifying Neuro-Developmental Issues (BCF)
By Brainchild Developmental Program
THE BRAINCHILD FOUNDATION COURSE: identifying neuro-developmental issues (BCF)

This course is suitable for all managers and supervisors who have a responsibility for providing high health and safety standards in the workplace. This qualification is applicable to any private or public sector working environment. Individuals working towards this qualification will learn that supervisors and managers have legal and moral obligations to ensure health and safety within a business and that these include obligations to employees, contractors, suppliers and members of the public.

Overview Internal auditing is an independent and objective activity to evaluate an organisation's internal operations. You'll learn how to initiate an audit, prepare and conduct audit activities, compile and distribute audit reports and complete follow-up activities. It is very important for the organisation to have a smooth flow of accounting as it plays a very important role in the development of the organisation. Financial Managers or any person who deals with Accounts need to see that the company accounts are very updated and are free from any risks that can become a problem during the time of Auditing. Objectives By the end of the course, participants will be able to: Efficiently dealing with senior leaders with confidence Effective Contribution and Strategically Analysing and Auditing towards business success Analysing and Evaluating as an effective internal audit leader How to manage key relationships with the audit committee Practical methods for managing the audit committee and senior management Describing the significance to help maximize the contribution to their organization

Tackling Financial Corruption
By Global Risk Alliance Ltd
This course will provide delegates involved in tackling the financial underpinning of corrupt behaviour with the knowledge, concepts and practices necessary to understand and mitigate illicit financial flows.

Selling with NLP (In-House)
By The In House Training Company
Take your sales people from average to high performance. Motivate and develop experienced sales professionals with some new insights and learning. Applying NLP principles, techniques and models, this workshop will introduce the core attitudes and behaviours that differentiate the excellent sales person from the average one. The programme will help participants: Understand and adopt the mindset and beliefs needed for sales excellence Build rapport and connect with buyers at a deeper and more personal level Recognise some of the thinking and language patterns that make each individual unique Ask powerful questions to further understand the unique world of the individual and how they make decisions Apply tools and techniques to empathise with clients - seeing things from their perspectives Tailor their sales approach to the individual buyer's style, and talk in their language Influence with integrity and sell to organisations and individuals successfully 1 Introduction Aims and objectives of the programme Personal introductions and objectives Workshop overview 2 An introduction to NLP and sales excellence with NLP An overview of NLP and applying it to selling The pillars of NLP The NLP model of communication The difference that makes the difference 3 Building enhanced rapport Defining rapport and why it is important when selling Going beyond the initial small talk Building relationships with individual decision-makers Matching and mirroring Levels of rapport 4 Understanding the buyer's personal buying map How we take in, filter and process information How we judge others based on our own experiences of the world The different ways in which we communicate when selling Recognising and understanding the language and thinking patterns of others Adapting your sales communication style to different buyers 5 Making sense of the buying process How we filter information through our senses Understanding how we see, hear and experience the world Visual, auditory and kinaesthetic buyers Listening for key insights What different buyers want from you to help them to buy Applying sensory awareness to the sales process 6 Successful sales mindset The connection between thoughts and actions The sales beliefs of excellence Identifying negative thoughts and beliefs that are holding you back How to change your mindset Adopting the sales beliefs of excellence 7 Powerful questions Reviewing and honing your questioning skills Understanding the questions that great sales people ask Avoiding assumptions Clean language questions Getting to the bottom of it - precision questions Turbo-charging how you qualify 8 Influencing with integrity Understanding empathy Stepping into the buyer's shoes Speaking the buyer's language Tailoring your sales approach to the individual Match, pace, lead - how to take your buyer with you 9 Putting it all together Personal learning summary and action plans

Search By Location
- Auditor Courses in London
- Auditor Courses in Birmingham
- Auditor Courses in Glasgow
- Auditor Courses in Liverpool
- Auditor Courses in Bristol
- Auditor Courses in Manchester
- Auditor Courses in Sheffield
- Auditor Courses in Leeds
- Auditor Courses in Edinburgh
- Auditor Courses in Leicester
- Auditor Courses in Coventry
- Auditor Courses in Bradford
- Auditor Courses in Cardiff
- Auditor Courses in Belfast
- Auditor Courses in Nottingham
