- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
65657 Courses
How to Let Go of Screen Time
By LivePlayLearn
Here are three reasons not to let go of screen time: 1/ It’s expensive. 2/ You might find yourself doing things that you don’t enjoy 3/ It can no longer be used as a babysitting or bargaining tool. I’m sure there are many more reasons why parents decide to restrict screen use and find it one of the hardest things to relinquish control over when they move to unschooling. It was certainly one of the last things that we let go off. We didn’t really explore releasing limits until my eldest was 5 year’s old (I think) We had probably relaxed things before that because it was easy when I was exhausted from having a tiny baby, toddler and young child with me all day long. We would all sit/ lie down/ sleep together in the living room in the afternoon whilst the TV was on. It was definitely used as a ‘babysitting tool’ whilst I rested. Honestly, this was ten years ago. We didn’t actually own a TV at the time and accessed streaming services on line. The children watched ten minute long cartoons and they didn’t automatically run onto the next episode so I had to wake up every ten minutes to select the next one! How things have changed in ten years!! Not everything is bad about lifting screen restrictions but like most things it does take consideration. I wouldn’t advice doing it just because it’s the unschooling way. Especially if you feel uncomfortable with the idea. Take your time. Do your research. Find out what unschooling families are doing instead (because it isn’t simply a case of lifting restrictions and leaving our children to it) For every reason you find not to do it, there is an unschooling reason to do it. It can be expensive but it doesn’t have to be and we can see it as an investment in our children’s education. You might find yourself playing apps or watching TV shows that you find frustrating or find dull but we relish the joy that our children get from spending time doing the things that they love. It can no longer be used as a babysitting tool or bargaining tool but it will deepen your relationship with your child and you can find other ways to meet your needs that don’t disconnect you from your child. This webinar will give you practical steps towards lifting screen restrictions in your family and prepare your for the difference that it will make in your home and your child's learning. This webinar is FREE for LPL Monthly Members along with a back catalogue of Unschooling Webinars, live webinars every month, and discounts on coaching and mentoring services. Sign up for just £20 per month. Yes! I want to join the LPL Monthly Membership

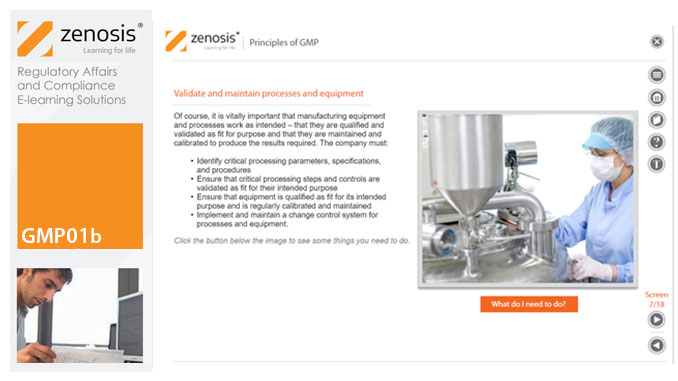
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
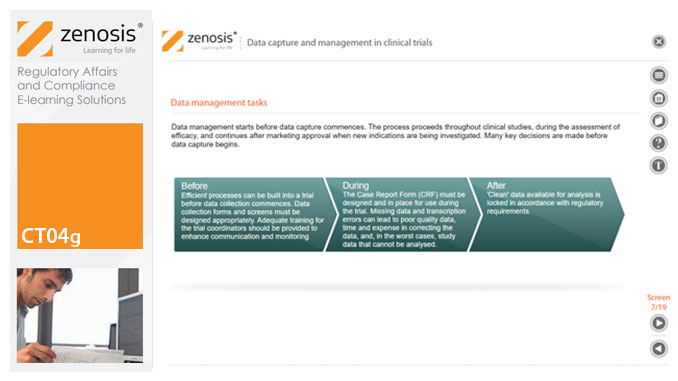
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
Low Demand Learning
By LivePlayLearn
Unschooling PDA children

Play is Serious Business
By LivePlayLearn
When children are able to choose how they spend their time, they inevitably choose to play because it is the basis for human learning. Can this be enough for a complete education?

Get the Practical CBT Clinical Will Template
By Practical CBT
Get the Practical CBT Clinical Will Template.

Moving From Control to Connection
By LivePlayLearn
Want to trust your child more but not sure where to start? This is a practical look at how we can increasingly move towards to more connected relationship with our children by increasingly trusting them with things in their own lives.

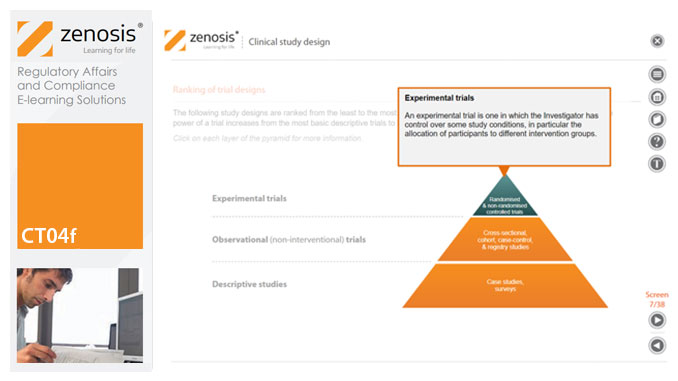
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
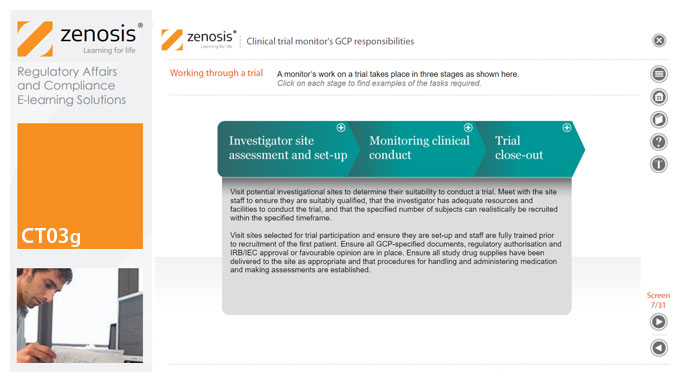
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
Report Writing When Your Child is in Burnout
By LivePlayLearn
Reporting to the LA when your child is in burnout

Search By Location
- Courses in London
- Courses in Birmingham
- Courses in Glasgow
- Courses in Liverpool
- Courses in Bristol
- Courses in Manchester
- Courses in Sheffield
- Courses in Leeds
- Courses in Edinburgh
- Courses in Leicester
- Courses in Coventry
- Courses in Bradford
- Courses in Cardiff
- Courses in Belfast
- Courses in Nottingham