- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Fast-Moving Consumer Goods (FMCG) & Retail Management - CPD Certified
4.7(47)By Academy for Health and Fitness
Unlock your path to success in the dynamic world of Fast-Moving Consumer Goods (FMCG) & Retail Management with our comprehensive 6-course bundle. As the demand for skilled Retail Managers continues to soar, with job openings growing by 7% annually (source: Bureau of Labor Statistics), our expert-led program equips you with the knowledge and skills to thrive in this booming industry. Don't miss this opportunity to accelerate your career - enrol now and take the first step towards becoming a retail management superstar! This Fast-Moving Consumer Goods (FMCG) & Retail Management Bundle Contains 6 of Our Premium Courses for One Discounted Price: Course 01: Product Management Course 02: Good Manufacturing Practice (GMP) Course 03: Retail Management Course 04: Quality Assurance (QA) Management Course 05: Purchasing and Procurement Course 06: Supply Chain Management Learning Outcomes: Master Product Management strategies for market success. Ensure compliance with Good Manufacturing Practice (GMP) standards. Lead and excel in Retail Management with confidence. Elevate Quality Assurance (QA) Management expertise. Optimise Purchasing and Procurement operations. Streamline Supply Chain Management for efficiency. Gain a holistic understanding of FMCG & Retail dynamics. Embark on a transformative journey through the Fast-Moving Consumer Goods (FMCG) & Retail Management world with our comprehensive 6-course bundle. Each course is carefully designed to empower you with essential skills and insights to thrive in this thriving industry. Product Management: Unlock the secrets of successful product strategies, from ideation to launch, and steer your brand towards market dominance. Good Manufacturing Practice (GMP): Ensure product quality and safety, complying with industry standards, and bolster consumer trust. Retail Management: Acquire the tools to lead, optimise, and innovate in the dynamic realm of retail, ensuring superior customer experiences. Quality Assurance (QA) Management: Become the guardian of product quality, guaranteeing excellence and compliance at every stage. Purchasing and Procurement: Master procurement strategies to cut costs and enhance the efficiency of your supply chain. Supply Chain Management: Streamline operations, minimise costs, and maximise value throughout your supply chain network. CPD 60 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Aspiring Product Managers Quality Control Professionals Retail Enthusiasts Procurement Specialists Requirements You will not need any prior background or expertise. Career path Product Manager: £35,000 - £75,000 Quality Assurance Manager: £30,000 - £65,000 Retail Manager: £25,000 - £60,000 Procurement Specialist: £30,000 - £65,000 Supply Chain Manager: £35,000 - £80,000 FMCG Industry Professional: Salary varies by role and experience. Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - £29 If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate

Boost Your Career By Enrolling In This Manufacturing & Product Management Bundle To Overcome Your Challenges! 6 in 1 Manufacturing & Product Management Bundle Improve your knowledge and enhance your skills to succeed with this Manufacturing & Product Management bundle. This Manufacturing & Product Management bundle is designed to build your competent skill set and enable the best possible outcome for your future. Our bundle is ideal for those who aim to be the best in their fields and are always looking to grow. This Manufacturing & Product Management Bundle Contains 6 of Our Premium Courses for One Discounted Price: Course 01: Modern Manufacturing Training Course 02: Good Manufacturing Practice (GMP) Course 03: Product Management Course 04: SAP S4HANA Controlling Level 2 Course 05: SAP Controlling (CO) - Product Costing S4HANA Course 06: Quality Assurance (QA) Manager All the courses under this Manufacturing & Product Management bundle are split into a number of expertly created modules to provide you with an in-depth and comprehensive learning experience. Upon successful completion of the Manufacturing & Product Management bundle, an instant e-certificate will be exhibited in your profile that you can order as proof of your new skills and knowledge. Stand out from the crowd and get trained for the job you want. With this comprehensive Manufacturing & Product Management bundle, you can achieve your dreams and train for your ideal career. This Manufacturing & Product Management bundle covers essential aspects in order to progress in your chosen career. Why Prefer Us for Manufacturing & Product Management? All-in-one package of 6 premium courses' Manufacturing & Product Management bundle Earn a certificate accredited by CPDQS. Get a free student ID card! (£10 postal charge will be applicable for international delivery) Globally Accepted Standard Lesson Planning Free Assessments with this Manufacturing & Product Management Bundle 24/7 Tutor Support available with this Manufacturing & Product Management Bundle Start your learning journey straightaway! This Manufacturing & Product Management's curriculum has been designed by Manufacturing & Product Management experts with years of Manufacturing & Product Management experience behind them. The Manufacturing & Product Management course is extremely dynamic and well-paced to help you understand Manufacturing & Product Management with ease. You'll discover how to master the Manufacturing & Product Management skill while exploring relevant and essential topics. Assessment Process Once you have completed all the courses in the Manufacturing & Product Management bundle, you can assess your skills and knowledge with an optional assignment. Our expert trainers will assess your assignment and give you feedback afterwards. CPD 60 CPD hours / points Accredited by CPD Quality Standards Who is this course for? This Manufacturing & Product Management bundle is suitable for everyone. Requirements You will not need any prior background or expertise. Career path This Manufacturing & Product Management bundle will allow you to kickstart or take your career in the related sector to the next stage. Certificates Digital certificate Digital certificate - Included Hard copy certificate Hard copy certificate - £29 If you are an international student, you will be required to pay an additional fee of 10 GBP for international delivery, and 4.99 GBP for delivery within the UK, for each certificate

Online Options
Show all 61Elevate your expertise with our comprehensive Good Manufacturing Practice (GMP) Training Course. Master the essentials of GMP across cosmetics, food, and pharmaceutical industries. Dive into cutting-edge topics like IT applications, contamination control, and maintenance strategies. Join us to foster a culture of quality, compliance, and excellence in manufacturing. Transform your career with industry-relevant knowledge and practical insights. Enroll now for a future where your skills drive the standards of safety and quality.

This course is designed to equip participants with comprehensive knowledge in Pharmaceutical Quality System and Good Manufacturing and Distribution Practices. It covers the entire lifecycle of pharmaceutical production, from initial design and development to distribution and compliance. The course includes real-world case studies and interactive tools. Course Objectives Career Launch: Equip those with little or no GMP/QA experience with the essential knowledge, practical skills, and industry support needed to launch a successful career. Comprehensive Foundation: Provide a strong foundational understanding of QA in the EU, UK, and US, preparing you for diverse opportunities in the global market

Computer System Validation Training Course (ONLINE). Extend Your Role to CSV Projects. Get Certified and Become a CSV Professional
By Getreskilled (UK)
Has the Computer System Validation Engineer left and you’ve been handed their responsibilities? Do the thoughts of your next audit fill you with dread? CSV can be frustrating but this program will show you how to manage electronic data in a regulated manufacturing/laboratory/clinical environment using the GAMP framework and ensure compliance with FDA’s 21 CFR Part 11, EU Annex 11 or other regulatory guidelines.

Introduction to Good Manufacturing Practice
By Research Quality Association
Course Information This course offers foundational guidance and practical support tailored for individuals operating within Good Manufacturing Practice (GMP) frameworks. Explore the fundamental prerequisites of a pharmaceutical quality system (PQS) and delve into the application of quality risk management (QRM) principles, aligning with current regulations and guidance. Gain insights into pivotal aspects such as requirements, roles, and responsibilities, encompassing change control, document management, and key documentation essential for effective implementation of GMP with a focus on regulatory inspections and common findings. Is this course for you? Ideal for professionals engaged in GMP across various sectors, including: Research and Development (R&D) Contract Manufacturing Organisations Manufacturing Units Quality Control (QC) Laboratories Auditing Roles. What will you learn? Event objectives - by the end of the course, delegates shall: Have an awareness of the basic requirements of GMP Be aware of UK and EU GMP Rules and Guidance and relevant publications Understand the roles and responsibilities associated with GMP Be able to contribute to and maintain quality documentation Have a basic understanding of product lifecycle and manufacturing Understand the requirements of GMP in the QC laboratory context Have a basic understanding of risk management and mitigation principles Understand the need for quality systems and quality assurance activities Be aware of common regulatory findings. Learning outcomes: delegates will be able to: Implement their role within GMP with confidence and knowledge of the principle requirements Contribute effectively to the GMP quality system and their organisation’s compliance Comprehend where their organisation’s activities sit within the larger GMP arena Know where to seek further information within the published rules and guidance, UK Legislation, European Commission Directives, ICH Guidance and other relevant publications, as well as via the internet. Tutors Tutors will be comprised of (click the photos for biographies): Louise Handy Director, Handy Consulting Ltd Programme Please note timings may be subject to alteration. Day 1 09:30 Introductions and Scope of the Course Understand the group requirements and the tutor's background and experience. 09:45 Background and Regulatory Environment Setting the scene, understanding the context, key legislation. 10:30 Principles of GMP Key points and requirements. 11:15 Break 11:30 Personnel and Responsibilities Management and staff, duties and accountabilities. 12:00 Overview of GMP Manufacturing Basics of the product life cycle. 12:30 Lunch 13:15 Risk Management Workshop Practical exploration of risk and mitigation activities. 14:30 QC Laboratories Activities and practicalities. 15:15 Break 15:30 Compliance Quality Assurance and Self Inspection. 16:15 Question Time A chance for questions on the practicalities of GMP. 16:30 Close of Course Extra Information Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 7 Points Development Level Learn

Retrain ONLINE for a Higher-Paying Job in the Pharma Manufacturing Industry in 10-Weeks
By Getreskilled (UK)
Average entry-level pharma roles pay 96% of NHS Band 5 and come with opportunities for overtime, shift allowance and additional benefits as well as huge scope for career progression and salary increases.

Equipment Validation Training Course (ONLINE) – For Starter Validation, CQV and C&Q Roles
By Getreskilled (UK)
Take our part-time online “Conversion Course into Pharmaceutical Manufacturing“ NO Previous Industry Experience or Science Qualifications Required

GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

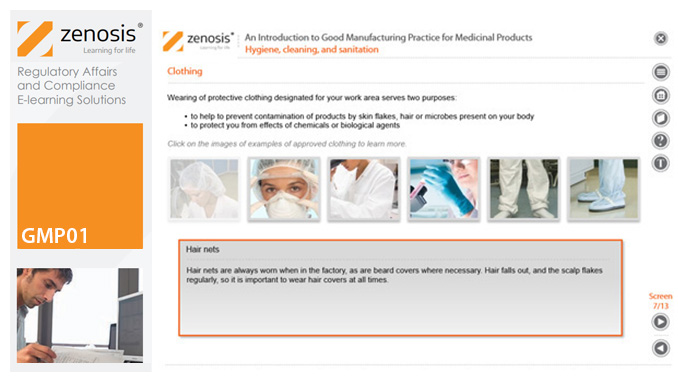
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
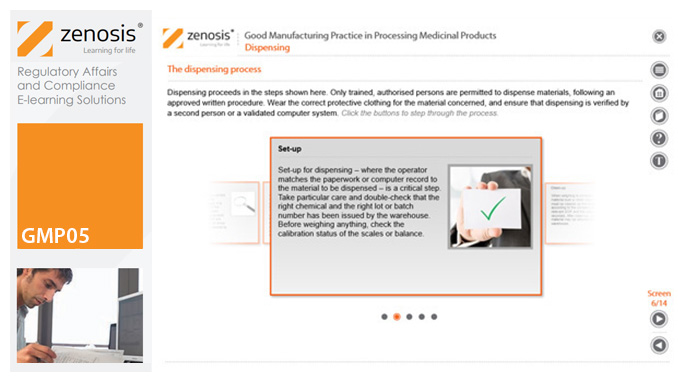
GMP05: Good Manufacturing Practice in Processing Medicinal Products
By Zenosis
Operations in the dispensary and on processing lines are at the heart of medicinal product manufacturing. This module describes how to carry out such operations in compliance with the requirements of Good Manufacturing Practice.
The Remote Auditing Course
By Research Quality Association
Course Information Join us for a two-day immersive course crafted to equip participants with an in-depth understanding of remote audit methodologies. Delve into the nuances between remote and face-to-face audits, dissecting their respective strengths and limitations. Through practical scenarios tailored for remote audit conduct, this course stands as an essential counterpart to our on-site audits course The Auditing Course. Who Should Attend: Applicable across various domains of regulated research and development, this course proves invaluable in contexts requiring a quality system for audit. Participants with firsthand audit experience stand to gain the most benefit from this programme. Expanding on Previous Learning: This course extends its relevance to all forms of audits and further amplifies concepts explored in RQA's suite of research quality assurance courses, including: 'Research Quality Assurance for Good Laboratory Practice,' 'Good Clinical Practice Auditing – Principles and Practice,' and 'Good Manufacturing Practice for Investigational Medicinal Products.' Benefits include improved: Understand the processes of planning, conducting, reporting and follow-up of audits Recognising the importance of personal approach in developing positive audit outcomes Ability to analyse evidence and present logical audit findings Appreciate the importance of audit in continuing improvement. This course is structured to encourage delegates to: Discuss and develop ideas Solve problems Exchange information. Tutors Tutors will be comprised of (click the photos for biographies): Andrew Waddell Founder Director, Tower Mains Ltd Rosemary Ichaba Senior QA Associate, Tower Mains Ltd Cate Ovington Director, The Knowlogy Group Ltd Jean McWilliam Associate Director, Alexion Programme Please note timings may be subject to alteration. Day 1 09:00 Course Registration 09:15 Welcome and Introductions 09:35 Introduction to Audits Delegates explore the range of audits which they have experienced, define the purpose of each audit type and establish which of those audits are performed to meet regulatory requirements. 10:05 Introduction to Remote Audits Presentation to introduce the major differences between face-to-face audits and remote audits. The major elements of audits will be examined to determine where there may be differences. 10:30 Break 10:45 Workshop 1 - Remote Audits This workshop will examine participants experience or understanding of the differences between face-to-face and remote audits. 11:15 Workshop 1 - Feedback 11:35 Audit Preparation The essential steps in preparation for audits will be discussed with emphasis on remote audits. 12:05 Workshop 2 - Remote Audit Preparation Factors relating to the preparation for remote audits will be considered in discussion groups. 12:35 Workshop 2 - Feedback 12:50 Lunch 13:30 Audit Logistics Preparation for the audit includes many arrangements other than the audit content. 14:00 Workshop 3 - Audit Logistics Delegates will discuss some of the issues that may occur during the set up and conduct of remote audits and how to resolve them. 14:30 Workshop 3 - Feedback 14:45 Break 15:00 The Opening Meeting Presentation regarding the importance and content of the opening meeting. 15:20 Workshop 4 - Opening Meeting Delegates will consider the practicalities of arranging and conducting an opening meeting. 15:45 Workshop 4 - Feedback 16:10 Audit Conduct Tools Practical advice on preparation of some of the tools that will be valuable in the conduct of audits. 16:40 Questions and Answers An overview of the first day and a chance to ask questions 17:00 Close of Day Day 2 09:00 Reflections on Day 1 Recap on the topics covered and a chance for delegates to raise points. 09:15 Gathering Evidence Presentation on challenges of gathering evidence and conducting interviews during remote audits. 09:45 Workshop 5 - Remote Interviews Scenarios of different remote interviews will be played and delegates assess the strengths and weaknesses of each approach. 10:15 Break 10:30 Creating Audit Findings Ideas regarding how to create good audit findings will be discussed during this short presentation. 10:50 Workshop 6 - Creating Audit Findings Delegates will be provided with examples of evidence from an audit. They will discuss these and create their audit findings for presentation at the closing meeting. 11:35 The Closing Meeting The content and conduct of the closing meeting will be discussed. 11:55 Workshop 7(a) - Preparing for the Closing Meeting The findings from workshop 6 will be reviewed and a Lead Auditor selected to provide feedback in workshop 7(b) 12:15 Workshop 7(b) - The Closing Meeting The Lead Auditor from each group will hold a meeting to present the results (role play). 12:45 Lunch 13:30 The Audit Report This presentation will cover not only the essential contents of the audit report but also how to write the report in a manner that will generate the most positive reaction. 14:00 Workshop 8 - The Audit Report Delegates will be provided with an audit report from an audit conducted remotely and will conduct a peer review of the report in their breakout groups. 15:00 Workshop 8 - Feedback 15:20 Break 15:35 Audit Closure Presentation on how to conclude the audit with reference to post-audit activities. 15:55 Open Forum 16:30 Close of Course Extra Information Course material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking You will need a stable internet connection, a microphone and a webcam.

Educators matching "Good Manufacturing Practice (GMP) Training Course"
Show all 2Search By Location
- Good Manufacturing Practice (GMP) Training Course Courses in London
- Good Manufacturing Practice (GMP) Training Course Courses in Birmingham
- Good Manufacturing Practice (GMP) Training Course Courses in Glasgow
- Good Manufacturing Practice (GMP) Training Course Courses in Liverpool
- Good Manufacturing Practice (GMP) Training Course Courses in Bristol
- Good Manufacturing Practice (GMP) Training Course Courses in Manchester
- Good Manufacturing Practice (GMP) Training Course Courses in Sheffield
- Good Manufacturing Practice (GMP) Training Course Courses in Leeds
- Good Manufacturing Practice (GMP) Training Course Courses in Edinburgh
- Good Manufacturing Practice (GMP) Training Course Courses in Leicester
- Good Manufacturing Practice (GMP) Training Course Courses in Coventry
- Good Manufacturing Practice (GMP) Training Course Courses in Bradford
- Good Manufacturing Practice (GMP) Training Course Courses in Cardiff
- Good Manufacturing Practice (GMP) Training Course Courses in Belfast
- Good Manufacturing Practice (GMP) Training Course Courses in Nottingham


