- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
9545 Courses
Professional Diploma in UX Design
By UX Design Institute
Become a Certified User Experience Professional Build a career in UX with the world’s only university credit-rated online UX course. Acquire the mindset, the skills and the confidence that make UX designers so valuable. A rewarding and meaningful career awaits. Why become a UX designer? Be in demand UX is a high-growth sector. The demand for UX designers far outstrips the supply of qualified professionals. Get well paid UX designers are highly paid. The average entry-level salary for a UX designer in the United Kingdom is £35,465 (PayScale, 2019) Love your job UX designers make an impact. They solve real-world problems using an exciting mix of research, design, technology and psychology. Study method Online, self-paced Duration 6 months Access to content 12 months Qualification Level 8 Diploma - User Experience Design Awarded by Glasgow Caledonian University Regulated by SQA Additional info Exam(s) / assessment(s) is included in price Tutor is available to students Description Duration: 6 months Get certified in six months following a flexible, structured learning path. Delivery: Online The course is delivered entirely online, including video modules, mentor-led webinars and support. Assessment: 2-hour exam Before getting certified, you'll be assessed during a two-hour exam taken online. Outcomes for you Think like a UX designer; adopt the mindset that sets them apart Showcase your knowledge with a portfolio of project work Speak with the confidence that comes from a true, deep understanding of UX Advance your career with job-ready skills Complete a university credit-rated course, valued by employers globally Become a Certified UX Professional Learn with us We’ve worked hard to design the best possible online learning experience for you. As well as an unmatched syllabus, our approach includes: Projects & portfolio Learn by doing with a series of real-world projects ideal for your UX portfolio. Mentors & webinars Be guided by our hand-picked, world-class mentors during monthly webinars. Structure & support Stay motivated with a structured programme supported by fellow students and our customer success team. What you’ll learn We’ve put together a university credit-rated curriculum that’s deep, rigorous and covers everything you need to know to become a certified UX professional and turbocharge your career. Module 01 - Introduction to UX design Module 02 - User research Module 03 - User goals Module 04 - Structure and navigation Module 05 - Interactions Module 06 - Design principles Module 07 - Design patterns Module 08 - Mobile Module 09 - Workflows Module 10 - Prototyping and handover Module 11 - AI and UX Module 12 - Creating your portfolio Module 13 - Career guidance Hub Tutorials for Figma Projects and portfolio - Projects that build into a portfolio Exam - 2-hour final exam Requirements Background You don’t need experience in design or technology to enrol in our courses, although having one or both is a definite bonus. Our students come from a diverse array of backgrounds, including project management, development, graphic design, product management, business analysis and so on. Commitment You do need to be motivated and committed. We set a high bar. Studying for one of our professional qualifications requires a certain amount of time, energy and focus. Our team will be there to support you along every step of the way but success will come as a result of your own diligence. Career path The average salary for entry level user experience designers is £28,000 The average salary for user experience designers with 1-5 years experience ranges from £29,000 to £50,000 The average salary for user experience managers/leads is £80,000 Information from LinkedIn Salary Reports, based on real jobs listings.

Launch your Detailing Career. Level One Motorbike is ideal for those just about to enter the detailing industry, or those who wish to reset, update and refresh their skills. The aim is to familiarise the candidate with the processes and maintenance of vehicles from a professional and business view for a new detailing career, teaching the foundations of correct vehicle cleaning up to the final familiarisation of entry-level dual-action machine polishers. Business practices and health and safety are explored covering areas such as pricing, marketing, target clientele, SDS and environmental conformity. This is much more than just a 'how to wash a vehicle' course, and gives your business the best possible start, optimising it for success. The aim of Level One Motorbike is ideal for those just about to, or have recently entered the motorbike detailing industry, or those who wish to reset and refresh their skills with a focus exclusively on motorbikes. The aim is to familiarise the candidate with the processes and maintenance of two-wheeled vehicles from a professional and business view, teaching the foundations of correct vehicle cleaning up to the final familiarisation of entry-level dual-action machine polishers. Business practices and health and safety are explored covering areas such as pricing, marketing, SDS and environmental conformity. The Level 1 Course is available as a motorbike focused, or alternatively our standard vehicle course. Alternatively, candidates can book an additional day on Motorcycle Detailing as a bolt-on to an automotive Level 1 Course here if they would like to learn both sets of skills. Along the way topics covered will be: Washing stages Wash media Environmental considerations Drying methods Bonded contamination and its removal Wheel care and maintenance Glass and hard surfaces Engine bay cleaning Vulnerable surfaces Efficiency and process PPE and safety Clothing and working practices Risk assessment Chemical knowledge Van set-ups Interior detailing Leather and soft surface care Fillers/glazing Dual action machine polishers intro Minor defect identification Paint types LSPs Final presentation Marketing Conflict resolution and customer care Financing Insurance Pricing and quotes Business Administration Memberships Social Media Manual handling Safe working practices Pre-work inspection All levels are accompanied by a full course booklet to jog your memory when needed. Practical assessment takes place as part of the original training session, at the end of the course you will be assigned a (manageable) series of case studies and exercises to complete over the period of 3 months to cement the knowledge. There is then a short externally assessed exam prior to the optional commencement of Level Two, to ensure there are no weak areas. After which the Level One accreditation is awarded and you can either progress or choose to practice the skills gained with ongoing support. Course Length 3 Days (0930 - 1600) Group Size One-to-One Location UK Detailing Academy, 2 Purlieus Barn, Ewen, Cirencester, GL7 6BY Experience / Qualification Open to all Refreshments or Lunch Refreshments included

Launch your Detailing Career. Are you looking to launch a detailing career and need to start on the right foot? Level One is ideal for those just about to enter the detailing industry, or those who wish to reset, update and refresh their skills. The aim is to teach you the foundations of vehicle care from assessment up to entry-level machine polishing, to have a successful and reputable business from the very start. Business practices and health and safety are explored covering areas such as pricing, marketing, target clientele, SDS and environmental conformity. This is much more than just a 'how to wash a car'. It gives your new detailing business the best possible start, optimising it for success and backed with a resource for any issues you may encounter. Complete course guide book, ongoing support and certification exam fee are all included in the cost. The Level 1 Course is available as either this standard vehicle course or alternatively a motorbike-focused course. You can also book the Level 1 + Motorbike course as a bolt-on day, if you wish to cover both aspects and open your target market even further. Available start dates are highlighted in the calendar below, all days are one-to-one so can be booked to suit your schedule. The aim of Level One is ideal for those just about to, or have recently entered the detailing industry, or those who wish to reset and refresh their skills. the aim is to familiarise the candidate with the processes and maintenance of vehicles from a professional and business view, teaching the foundations of correct vehicle cleaning up to the final familiarisation of entry-level dual action machine polishers. Business practices and health and safety are explored covering areas such as pricing, marketing, SDS and environmental conformity. The Level 1 Course is available as either a car or motorcycle course. Alternatively, candidates can book an additional day on Motorcycle Detailing as a bolt-on to an automotive Level 1 Course here. Modules covered on the course are: Washing stages Wash media Environmental considerations Drying methods Bonded contamination and its removal Wheel care and maintenance Glass and hard surfaces Engine bay cleaning Vulnerable surfaces Efficiency and process PPE and safety Clothing and working practices Risk assessment Chemical knowledge Van set-ups Interior detailing Leather and soft surface care Fillers/glazing Dual action machine polishers intro Minor defect identification Paint types LSPs Final presentation Marketing Conflict resolution and customer care Financing Insurance Pricing and quotes Business Administration Memberships Social Media Manual handling Safe working practices Pre-work inspection All levels are accompanied by a full course booklet to jog your memory when needed. Practical assessment takes place as part of the original training session, at the end of the course you will be assigned a (manageable) series of case studies and exercises to complete over 3 months to cement the knowledge. There is then a short externally assessed exam before the optional commencement of Level Two, to ensure there are no weak areas. After which the Level One accreditation is awarded and you can either progress or choose to practice the skills gained with ongoing support. Course Length 3 Days (0930 - 1600) Group Size One-to-One Location UK Detailing Academy, 2 Purlieus Barn, Ewen, Cirencester, GL7 6BY Experience / Qualification Open to all Refreshments or Lunch Refreshments included

AgilePM Practitioner
By IIL Europe Ltd
AgilePM® Practitioner This course offers preparation for the Practitioner-level examination to gain the APMG-International™ / Agile Project Management Practitioner Certification. Agile Project Management (AgilePM) is the result of collaboration between APMG-International and The DSDM Consortium. DSDM (Dynamic Systems Development Method) is the longest-established Agile method, launched in 1995, and is the only Agile method to focus on the management of Agile projects. The method has evolved over the years and the DSDM Agile Project Framework is the latest version of which AgilePM is a subset. DSDM has always operated predominantly in the corporate environment and has consistently demonstrated its ability to successfully work with and complement existing corporate processes. APMG-International is a global Examination Institute accredited by The APM Group Ltd. It is one of the Examination Institutes accredited by AXELOS. APMG-International has regional offices located in Australia, China, Denmark, Germany, the Netherlands, Malaysia, the United States, and the United Kingdom. Their portfolio of qualifications includes the Best Practice qualifications of ITIL®, PRINCE2®, MSP®, M_o_R®, and P3O®. AgilePM is one of their specialist management qualifications, which also include Change Management and Service Catalogue. The course covers all the Practitioner elements of the AgilePM Handbook v2 with: Clear explanations of the method and practical examples provided by your course tutor Sample exam paper for the Practitioner-level exams to enrich your knowledge and understanding A case study to allow you to practice the application of the method to an agile project The Traditional Classroom option includes the Practitioner exam to provide you with the right opportunity to verify your new skill set by way of a professional qualification The Virtual Classroom option includes a Practitioner exam voucher to allow you to choose the date and time of your online exam to verify your new skill set by way of a professional qualification What You Will Learn You will learn how to: Identify and apply the concepts, tools, and techniques described in Section 2 (Digging Deeper) of the APMG-International's Agile Project Management Handbook (v2.0) to agile projects Tailor and customize AgilePM to suit the needs of different projects Use AgilePM in conjunction with other project management methods such as PRINCE2® Prepare yourself for the Practitioner exam in AgilePM Roles and Responsibilities - The PM View The roles Key project manager relationships Agile Project Management - Through the Lifecycle The DSDM process and the project lifecycle Project management focus phase by phase The Effective Use of Products The products Deliver on Time - Combining MoSCoW & Timeboxing Ensuring effective prioritisation Bringing MoSCoW and timeboxing together People, Teams, and Interactions Effective communication Collaboration Requirements and User Stories What is a requirement? User stories Estimating - How and When Coping with uncertainty Estimating through the lifecycle Project Planning through the Lifecycle Planning in a DSDM project Planning activities phase by phase Quality - Never Compromise Quality What do we mean by quality? Solution and process quality Risk Management Project risk How DSDM helps mitigate project risk Tailoring the Approach The project approach questionnaire Summary and Next Steps

AgilePM Practitioner: In-House Training
By IIL Europe Ltd
AgilePM® Practitioner: In-House Training This course offers preparation for the Practitioner-level examination to gain the APMG-International™ / Agile Project Management Practitioner Certification. Agile Project Management (AgilePM) is the result of collaboration between APMG-International and The DSDM Consortium. DSDM (Dynamic Systems Development Method) is the longest-established Agile method, launched in 1995, and is the only Agile method to focus on the management of Agile projects. The method has evolved over the years and the DSDM Agile Project Framework is the latest version of which AgilePM is a subset. DSDM has always operated predominantly in the corporate environment and has consistently demonstrated its ability to successfully work with and complement existing corporate processes. APMG-International is a global Examination Institute accredited by The APM Group Ltd. It is one of the Examination Institutes accredited by AXELOS. APMG-International has regional offices located in Australia, China, Denmark, Germany, the Netherlands, Malaysia, the United States, and the United Kingdom. Their portfolio of qualifications includes the Best Practice qualifications of ITIL®, PRINCE2®, MSP®, M_o_R®, and P3O®. AgilePM is one of their specialist management qualifications, which also include Change Management and Service Catalogue. The course covers all the Practitioner elements of the AgilePM Handbook v2 with: Clear explanations of the method and practical examples provided by your course tutor Sample exam paper for the Practitioner-level exams to enrich your knowledge and understanding A case study to allow you to practice the application of the method to an agile project The Traditional Classroom option includes the Practitioner exam to provide you with the right opportunity to verify your new skill set by way of a professional qualification The Virtual Classroom option includes a Practitioner exam voucher to allow you to choose the date and time of your online exam to verify your new skill set by way of a professional qualification What You Will Learn You will learn how to: Identify and apply the concepts, tools, and techniques described in Section 2 (Digging Deeper) of the APMG-International's Agile Project Management Handbook (v2.0) to agile projects Tailor and customize AgilePM to suit the needs of different projects Use AgilePM in conjunction with other project management methods such as PRINCE2® Prepare yourself for the Practitioner exam in AgilePM Roles and Responsibilities - The PM View The roles Key project manager relationships Agile Project Management - Through the Lifecycle The DSDM process and the project lifecycle Project management focus phase by phase The Effective Use of Products The products Deliver on Time - Combining MoSCoW & Timeboxing Ensuring effective prioritisation Bringing MoSCoW and timeboxing together People, Teams, and Interactions Effective communication Collaboration Requirements and User Stories What is a requirement? User stories Estimating - How and When Coping with uncertainty Estimating through the lifecycle Project Planning through the Lifecycle Planning in a DSDM project Planning activities phase by phase Quality - Never Compromise Quality What do we mean by quality? Solution and process quality Risk Management Project risk How DSDM helps mitigate project risk Tailoring the Approach The project approach questionnaire Summary and Next Steps

Certified Cisco Networking for Beginners with Simulators and Exams
By Hudson
This course bundle is aimed at absolute beginners to IT who want to start a career in Cisco networking or just develop their skills in this area. The course assumes you have no technical knowledge of IT whatsoever. To understand Cisco networking and practice it at a technical level, you must first possess an understanding of basic IT literacy as well as certain technologies, which you will be taught through this course. Through this course, you will gain a series of globally recognised networking certifications from CompTIA and Cisco. You will also possess a set of networking skills you can apply to a vast range of IT job roles. From the beginning, we teach you about basic IT literacy, basic software features and functions, basic networking, operating systems, and basic security threats. From there, the course steps up a notch exploring these areas in more depth. As you progress through the various stages, your knowledge of networking will gradually begin to develop. The course bundle is comprised of five separate courses in total. Once of which is skills based only with no exam, and four certification courses with a final exam after each course. The final course being the Cisco Certified Networking Associate (CCNA). The first course (CompTIA IT Fundamentals) provides a soft introduction to basic IT terminology, concepts and technology. To help you grasp the concepts and develop the skills within the course, simulators are also included. These allow you to practice your networking skills learnt on the course. In the IT world, these simulators are otherwise known as ‘Live Labs’.

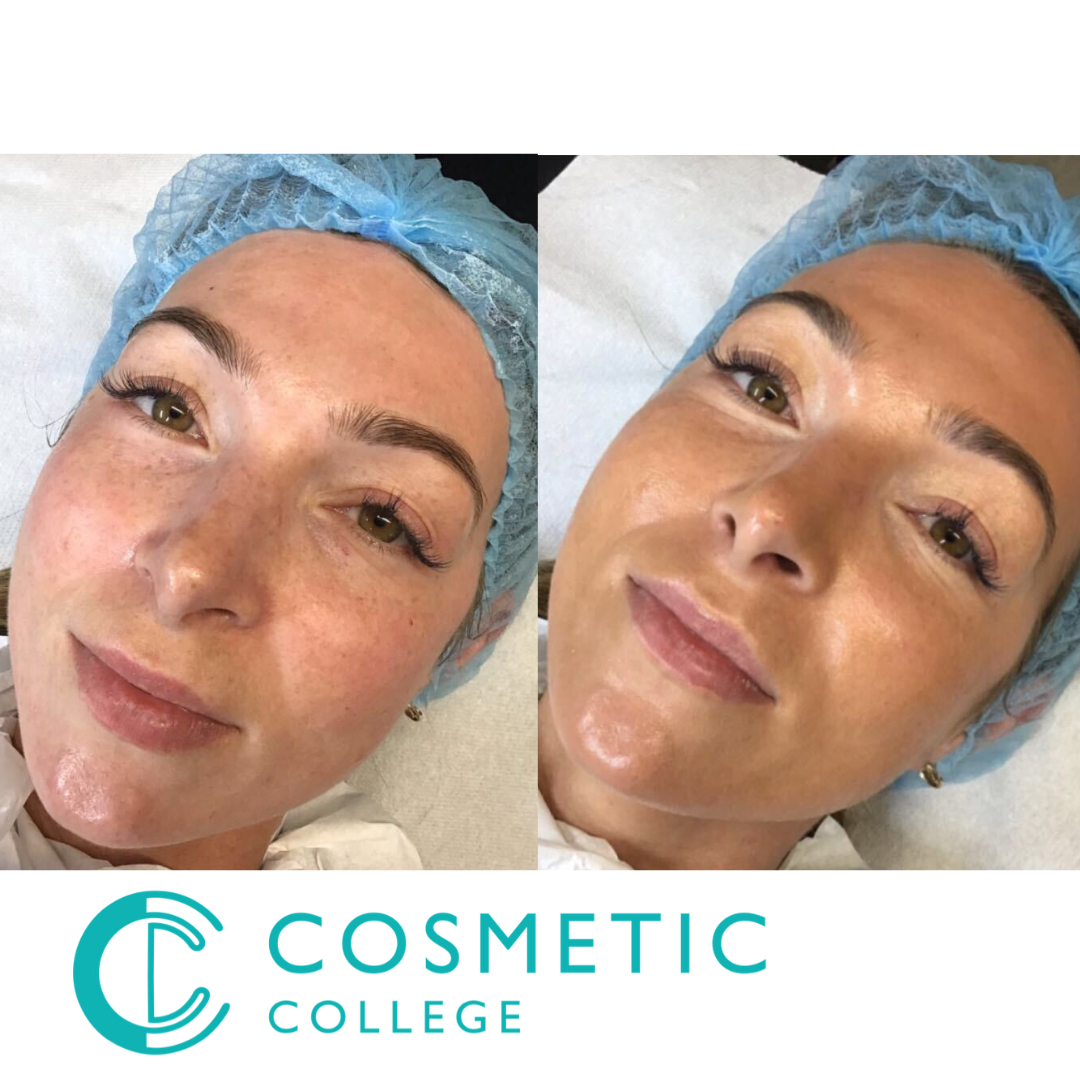
BB Glow Foundation Training
By Cosmetic College
The BB Glow Facial is one of the newer facial treatments to reach the beauty market and is becoming an increasingly popular choice for many clients. A BB Glow Foundation Facial is a highly effective micro-needling treatment with immediate, lasting effects. This safe and intensive skin treatment can improve the facial tone and smooth out imperfections, freckles, and wrinkles. The treatment goal of using the micro-needle therapy system is to lighten and smooth the face's skin tone. In this course, you will learn how to create a semi-permanent foundation lasting for months, which hides facial imperfections. Learn the newest mesotherapy treatment loved by the world, which can also be available in your beauty salon. Course Entry Requirements: This course is available for those that meet at least one of the following: Minimum 18 years NVQ Level 2 Beauty or equivalent desirable Good command of English Needling accredited training Course Pre-Study/Practical & Length: 20 hours of pre-study E-learning and 1 practical day Course Agenda This courses theory knowledge is delivered through our interactive e-learning training portal and completed with either: One day onsite training One day Zoom based training All courses are kept to a reduced size, with a maximum of six attendees per course. Areas covered in this course: Anatomy and physiology of face and skin The choice of colour for the type of skin Written documentation (treatment card) Photographic documentation The principles of hygiene Purpose of the treatment and the obtained effect Assessment of customer expectations Contraindications for BB Glow treatments Preparation of clients and positions for the procedure Application rules for anaesthesia Possible complications after treatments Types of pigments Insurance Practice: Practice on models Demonstration of the procedure on the model Enrolling in our BB Glow Foundation Training Course offers numerous benefits for students, as well as significant advantages for clients and potential earning potential. Let's explore these benefits in detail: Benefits for Students: Expertise in BB Glow Foundation: Our training course equips students with the knowledge and skills to master the BB Glow Foundation technique. You will learn the latest application techniques, colour theory, product selection, and client consultation. This specialised training will set you apart as a skilled BB Glow artist. Practical Hands-on Experience: We prioritise practical learning by providing extensive hands-on training opportunities. Under the guidance of experienced instructors, you will have the chance to practice the BB Glow Foundation technique on models. This practical experience will enhance your confidence and proficiency in delivering excellent results. Professional Advancement: By enrolling in our BB Glow Foundation Training Course, you are investing in your professional advancement. You will expand your skill set, stay updated with industry standards, and gain expertise in the popular BB Glow technique. This will enhance your professional reputation and open doors to new career opportunities. Benefits for Clients: Flawless and Radiant Skin: BB Glow Foundation is a popular semi-permanent makeup technique that creates the appearance of flawless, radiant skin. By enrolling in our training course, you will be able to offer this highly sought-after treatment to your clients. They will benefit from a semi-permanent foundation that evens out skin tone, hides imperfections, and provides a natural-looking glow. Time-saving Solution: Clients who opt for BB Glow Foundation can save time in their daily makeup routine. The semi-permanent nature of the treatment eliminates the need for daily foundation application, saving clients valuable time and effort. They can wake up with a fresh-faced look and feel confident throughout the day. Customised Results: As a trained BB Glow artist, you will have the expertise to customise the treatment to suit each client's skin tone and preferences. By selecting the appropriate pigments and adjusting the intensity, you can achieve personalised results that enhance the client's natural beauty. Clients will appreciate the tailored approach and individualized outcomes. Earning Potential: By offering BB Glow Foundation as part of your services, you can significantly increase your earning potential. The popularity of BB Glow treatments has been growing, and clients are willing to invest in achieving flawless, long-lasting skin. As a skilled BB Glow artist, you can attract a larger clientele and set competitive pricing for your services. Satisfied clients are more likely to become repeat customers and refer others, further boosting your earning potential. Enrolling in our BB Glow Foundation Training Course will not only benefit you as a student but also provide valuable advantages for your clients and potential earning potential in the beauty industry. Begin your journey toward professional excellence and financial success by enrolling today. Frequently Asked Questions (FAQ) about our BB Glow Foundation Training Course: Is this course suitable for beginners or those with prior experience? Our BB Glow Foundation Training Course is designed for both beginners and experienced professionals in the beauty industry. Whether you are new to semi-permanent makeup or already have a background in the field, our course will provide you with the necessary knowledge and skills to master the BB Glow Foundation technique. What qualifications or prerequisites do I need to enrol in the course? No specific qualifications are required to enrol in our BB Glow Foundation Training Course. However, a passion for the beauty industry and a desire to learn and improve your skills are highly recommended. Our course is open to anyone who wants to gain expertise in the BB Glow technique. Will I receive a certification upon completion? Yes, upon successfully completing our training course, you will receive a certification in BB Glow Foundation. This certification recognises your skills and expertise in performing the BB Glow technique. It can enhance your professional credibility and assist you in building your career in the beauty industry. What topics are covered in the course curriculum? Our course curriculum covers a wide range of topics related to BB Glow Foundation, including skin anatomy and physiology, product knowledge, colour theory, sanitation and hygiene, consultation and client assessment, application techniques, aftercare, and touch-up procedures. The curriculum is designed to provide a comprehensive understanding of the technique and its application. Are there any hands-on training opportunities? Absolutely! Our BB Glow Foundation Training Course includes extensive hands-on training sessions. You will have the opportunity to practice the BB Glow technique on models under the guidance of experienced instructors. This practical experience is essential to develop your skills and confidence in performing the treatment effectively. Will I have access to ongoing support after completing the course? Yes, we provide ongoing support to our students even after they complete the training course. Our instructors and support staff are available to answer any questions, provide guidance, and offer assistance as you embark on your career in BB Glow Foundation. We aim to support your continued growth and success. Are there financing options available for the course? We offer flexible payment options and financing plans to make our BB Glow Foundation Training Course more accessible. Please reach out to our admissions team for detailed information on available payment options and financing plans. If you have any additional questions or require further clarification, please feel free to contact us. We are here to assist you throughout your training journey and beyond. Course Benefits Benefits for Students Expertise in BB Glow Foundation: Our training course equips students with the knowledge and skills to master the BB Glow Foundation technique. You will learn the latest application techniques, colour theory, product selection, and client consultation. This specialised training will set you apart as a skilled BB Glow artist. Practical Hands-on Experience: We prioritise practical learning by providing extensive hands-on training opportunities. Under the guidance of experienced instructors, you will have the chance to practice the BB Glow Foundation technique on models. This practical experience will enhance your confidence and proficiency in delivering excellent results. Professional Advancement: By enrolling in our BB Glow Foundation Training Course, you are investing in your professional advancement. You will expand your skill set, stay updated with industry standards, and gain expertise in the popular BB Glow technique. This will enhance your professional reputation and open doors to new career opportunities. Benefits for Clients Flawless and Radiant Skin: BB Glow Foundation is a popular semi-permanent makeup technique that creates the appearance of flawless, radiant skin. By enrolling in our training course, you will be able to offer this highly sought-after treatment to your clients. They will benefit from a semi-permanent foundation that evens out skin tone, hides imperfections, and provides a natural-looking glow. Time-saving Solution: Clients who opt for BB Glow Foundation can save time in their daily makeup routine. The semi-permanent nature of the treatment eliminates the need for daily foundation application, saving clients valuable time and effort. They can wake up with a fresh-faced look and feel confident throughout the day. Customised Results: As a trained BB Glow artist, you will have the expertise to customise the treatment to suit each client's skin tone and preferences. By selecting the appropriate pigments and adjusting the intensity, you can achieve personalised results that enhance the client's natural beauty. Clients will appreciate the tailored approach and individualised outcomes. Earning Potential By offering BB Glow Foundation as part of your services, you can significantly increase your earning potential. The popularity of BB Glow treatments has been growing, and clients are willing to invest in achieving flawless, long-lasting skin. As a skilled BB Glow artist, you can attract a larger clientele and set competitive pricing for your services. Satisfied clients are more likely to become repeat customers and refer others, further boosting your earning potential. Enrolling in our BB Glow Foundation Training Course will not only benefit you as a student but also provide valuable advantages for your clients and potential earning potential in the beauty industry. Begin your journey toward professional excellence and financial success by enrolling today. Frequently Asked Questions Where is the Cosmetic College The Cosmetic College is located at: 3 Locks Court, 429 Crofton Road, Orpington, BR6 8NL How can I book? We have a few options for you to book. You can book by selecting an available training date above here on our website, by contacting us through email at hello@cosmetic.college or by contacting us on 0333 015 5117. Is a deposit required to book? All enrolments are charged an administration fee which is non-refundable. When you enrol you can elect to pay a deposit of 10% plus the administration fee or pay the total training course in full. We have full details of the terms and conditions of training course enrolments here Is this course suitable for beginners or those with prior experience? Our BB Glow Foundation Training Course is designed for both beginners and experienced professionals in the beauty industry. Whether you are new to semi-permanent makeup or already have a background in the field, our course will provide you with the necessary knowledge and skills to master the BB Glow Foundation technique. Will I receive a certification upon completion? Yes, upon successfully completing our training course, you will receive a certification in BB Glow Foundation. This certification recognises your skills and expertise in performing the BB Glow technique. It can enhance your professional credibility and assist you in building your career in the beauty industry. What topics are covered in the course curriculum? Our course curriculum covers a wide range of topics related to BB Glow Foundation, including skin anatomy and physiology, product knowledge, colour theory, sanitation and hygiene, consultation and client assessment, application techniques, aftercare, and touch-up procedures. The curriculum is designed to provide a comprehensive understanding of the technique and its application. Are there any hands-on training opportunities? Absolutely! Our BB Glow Foundation Training Course includes extensive hands-on training sessions. You will have the opportunity to practice the BB Glow technique on models under the guidance of experienced instructors. This practical experience is essential to develop your skills and confidence in performing the treatment effectively. Will I have access to ongoing support after completing the course? Yes, we provide ongoing support to our students even after they complete the training course. Our instructors and support staff are available to answer any questions, provide guidance, and offer assistance as you embark on your career in BB Glow Foundation. We aim to support your continued growth and success. Are there financing options available for the course? We offer flexible payment options and financing plans to make our BB Glow Foundation Training Course more accessible. Please reach out to our admissions team for detailed information on available payment options and financing plans. If you have any additional questions or require further clarification, please feel free to contact us. We are here to assist you throughout your training journey and beyond.
Advanced Certified Scrum Product Owner: In-House Training
By IIL Europe Ltd
Advanced Certified Scrum Product Owner® (A-CSPO®): In-House Training All Advanced CSPO courses are taught by Educators approved by the Scrum Alliance. Taking an Advanced CSPO course, meeting the learning objectives, and accepting the license agreement designates you as an Advanced Certified Scrum Product Owner. Please review your trainer's course description below to determine which learning objectives this course satisfies. What you will Learn You'll learn to: Manage multiple business initiatives from competing stakeholders Clearly order and express Product Backlog items Define a clear product vision that ensures your product remains focused on the features your customers and end users will actually use Communicate effectively with various stakeholder groups to achieve alignment Identify the crucial opportunities and avoid wasting time Define and validate business value Increase your credibility as a product expert and become recognized as a person who delivers real business results Benefits Build on your foundational knowledge with enhanced implementation skills Distinguish yourself in the global marketplace Stand out in your industry as a member of the Scrum Alliance globally-recognized community Show advanced value to your employer (or potential employer) as a highly trained Agile professional

Global Project Management: On-Demand
By IIL Europe Ltd
Global Project Management: On-Demand In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. In today's increasingly global environment, managing a project with customers and support organizations spread across multiple countries and continents is a major challenge. From identifying stakeholders and gathering requirements, to planning, controlling, and executing the project, the basic logistics of a global project present their own standard challenges. However, with additional cultural, language-based, and regional elements, global projects involve more complexities than teams often realize. There are unique communication needs, cultural awareness elements, varying customs and work expectations, and critical legal differences to consider. In this course, you will dig deeper-and differently-into project management processes, tools, and techniques, developing the ability to see them through the lens of global and cultural project impacts. This will leverage you to problem solve differently on global projects, prevent problems, and ensure success. The goal is for you to effectively navigate the challenges of leading projects with multi-regional footprints and globally diverse sets of stakeholders. What you Will Learn At the end of this program, you will be able to: Determine when a project meets the criteria of being a true global one Articulate global project needs based on the project grid and framework Identify and analyze global project stakeholders Recognize cultural differences and articulate how they impact project work Determine global project estimating, scheduling, and staffing challenges Assess global project risks and develop problem-solving responses Analyze complex cultural situations and align optimal project communication and negotiation tools and techniques Apply best practices for conducting virtual team work and mitigating virtual challenges Evaluate ways to control for global project scope, cost, and procurement Align customer management best practices with global customer needs Implement key global project closing activities Foundation Concepts What is a global project? What makes a global project different? A global project management framework Initiating the Global Project Launching a global project Respecting cultural differences Identifying and analyzing stakeholders Developing the communications plan Defining the ideal global project manager Crafting a global project charter Planning the Global Project Gathering requirements for a global project Defining the scope, region by region Estimating and scheduling for global projects Staffing the global project Developing the global risk management plan Executing the Global Project Managing global stakeholder expectations Embracing cultural diversity Honing global negotiation techniques Procuring goods and services on a global basis Managing global legal and regulatory issues at the micro and macro level Monitoring and Controlling the Global Project Status reporting Virtual communication Cost control Schedule control Scope control Customer satisfaction Closing the Global Project Contract closure at the macro and micro levels Administrative closure with global reach Lessons learned

This half-day Suicide First Aid Lite training course gives learners the knowledge and tools to understand that suicide is one of the most preventable deaths and some basic skills can help someone with thoughts of suicide stay safe from their thoughts and stay alive.

Search By Location
- GL Courses in London
- GL Courses in Birmingham
- GL Courses in Glasgow
- GL Courses in Liverpool
- GL Courses in Bristol
- GL Courses in Manchester
- GL Courses in Sheffield
- GL Courses in Leeds
- GL Courses in Edinburgh
- GL Courses in Leicester
- GL Courses in Coventry
- GL Courses in Bradford
- GL Courses in Cardiff
- GL Courses in Belfast
- GL Courses in Nottingham