- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
1327 Courses
Gas Lift Design & Optimization using NODAL Analysis
By EnergyEdge - Training for a Sustainable Energy Future
About this training course Gas-lift is one of the predominant forms of artificial lift used for lifting liquids from conventional, unconventional, onshore and offshore assets. Gas-lift and its various forms (intermittent lift, gas-assisted plunger lift) allows life of well lift-possibilities when selected and applied properly. This 5-day training course is designed to give participants a thorough understanding of gas-lift technology and related application concepts. This training course covers main components such as application envelope, relative strengths and weaknesses of gas-lift and its different forms like intermittent lift, gas-assisted plunger lift. Participants solve examples and class problems throughout the course. Animations and videos reinforce the concepts under discussion. Unique Features: Hands-on usage of SNAP Software to solve gas-lift exercises Discussion on digital oil field Machine learning applications in gas-lift optimization Training Objectives After the completion of this training course, participants will be able to: Understand the fundamental theories and procedures related to Gas-Lift operations Easily recognize the different components of the gas-lift system and their basic structural and operational features Be able to design a gas-lift installation Comprehend how digital oilfield tools help address ESP challenges Examine recent advances in real-time approaches to the production monitoring and lift management Target Audience This training course is suitable and will greatly benefit the following specific groups: Production, reservoir, completion, drilling and facilities engineers, analysts, and operators Anyone interested in learning about implications of gas-lift systems for their fields and reservoirs Course Level Intermediate Advanced Training Methods The training instructor relies on a highly interactive training method to enhance the learning process. This method ensures that all participants gain a complete understanding of all the topics covered. The training environment is highly stimulating, challenging, and effective because the participants will learn by case studies which will allow them to apply the material taught in their own organization. Course Duration: 5 days in total (35 hours). Training Schedule 0830 - Registration 0900 - Start of training 1030 - Morning Break 1045 - Training recommences 1230 - Lunch Break 1330 - Training recommences 1515 - Evening break 1530 - Training recommences 1700 - End of Training The maximum number of participants allowed for this training course is 20. This course is also available through our Virtual Instructor Led Training (VILT) format. Prerequisites: Understanding of petroleum production concepts. Each participant needs a laptop/PC for solving class examples using software to be provided during class. Laptop/PC needs to have a current Windows operating system and at least 500 MB free disk space. Participants should have administrator rights to install software. Trainer Your expert course leader has over 35 years' work-experience in multiphase flow, artificial lift, real-time production optimization and software development/management. His current work is focused on a variety of use cases like failure prediction, virtual flow rate determination, wellhead integrity surveillance, corrosion, equipment maintenance, DTS/DAS interpretation. He has worked for national oil companies, majors, independents, and service providers globally. He has multiple patents and has delivered a multitude of industry presentations. Twice selected as an SPE distinguished lecturer, he also volunteers on SPE committees. He holds a Bachelor's and Master's in chemical engineering from the Gujarat University and IIT-Kanpur, India; and a Ph.D. in Petroleum Engineering from the University of Tulsa, USA. Highlighted Work Experience: At Weatherford, consulted with clients as well as directed teams on digital oilfield solutions including LOWIS - a solution that was underneath the production operations of Chevron and Occidental Petroleum across the globe. Worked with and consulted on equipment's like field controllers, VSDs, downhole permanent gauges, multiphase flow meters, fibre optics-based measurements. Shepherded an enterprise-class solution that is being deployed at a major oil and gas producer for production management including artificial lift optimization using real time data and deep-learning data analytics. Developed a workshop on digital oilfield approaches for production engineers. Patents: Principal inventor: 'Smarter Slug Flow Conditioning and Control' Co-inventor: 'Technique for Production Enhancement with Downhole Monitoring of Artificially Lifted Wells' Co-inventor: 'Wellbore real-time monitoring and analysis of fracture contribution' Worldwide Experience in Training / Seminar / Workshop Deliveries: Besides delivering several SPE webinars, ALRDC and SPE trainings globally, he has taught artificial lift at Texas Tech, Missouri S&T, Louisiana State, U of Southern California, and U of Houston. He has conducted seminars, bespoke trainings / workshops globally for practicing professionals: Companies: Basra Oil Company, ConocoPhillips, Chevron, EcoPetrol, Equinor, KOC, ONGC, LukOil, PDO, PDVSA, PEMEX, Petronas, Repsol, , Saudi Aramco, Shell, Sonatrech, QP, Tatneft, YPF, and others. Countries: USA, Algeria, Argentina, Bahrain, Brazil, Canada, China, Croatia, Congo, Ghana, India, Indonesia, Iraq, Kazakhstan, Kenya, Kuwait, Libya, Malaysia, Oman, Mexico, Norway, Qatar, Romania, Russia, Serbia, Saudi Arabia, S Korea, Tanzania, Thailand, Tunisia, Turkmenistan, UAE, Ukraine, Uzbekistan, Venezuela. Virtual training provided for PetroEdge, ALRDC, School of Mines, Repsol, UEP-Pakistan, and others since pandemic. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Process Control and Instrumentation
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course This course will begin with a presentation of topics to familiarize Process and Instrumentation Engineers with procedures and practices involved in the choice of sensors related to the measurement of temperature, pressure, level and flow in relation to single-phase flows. It will provide guidance on the optimum commercially available devices through a detailed comparison of their relative merits. At the heart of this course is sensor calibration which is a crucial element for these topics. The course will also examine the various types of flow control valve, including Globe, Slide, Needle, Eccentric plug and Ball valves and their characteristics in industrial application, while focusing on the problems of Cavitation and Flashing and methods to minimise or eradicate these issues. With the use of examples, industry case studies and a wide range of videos, this course will also cover all aspects of proportional (P), derivative (D) and integral (I) control. In particular, it will address the advantages and disadvantages of PI and PID control. It will also describe Cascade, Feed forward, Split Range, Override and Ratio Control techniques. Training Objectives By attending this course, participants will acquire the following knowledge and skills: Apply an in-depth knowledge to the measurement of temperature, pressure, level and flow as well as to the fluid mechanics of pipe flows Assess the advantages and disadvantages of the major flowmeter types including the differential pressure, rotary positive displacement, rotary-inferential, electromagnetic, ultrasonic and Coriolis mass flowmeters to determine the optimum choice for a given application Make a considered judgement of the choice of fluid level measurement devices Understand the various types of flow calibration, metering systems and provers Carry out tank measurement and tank calibration methods and to calculate net sellable quantities Discuss valve characteristics & trim selection and illustrate the process of control valve sizing Explain the terms Open and Closed loop Define Process Variable, Measured Variable, Set Point and Error Define Direct and Reverse controller actions Explain the terms Process Lag, Measurement Lag, Transmission Lag, and Response Lag and their effect on controllability Explain ON/ OFF Control and the inherent disadvantages Explain Proportional Control, Offset, Gain and Proportional Band and the advantages and disadvantages of Proportional only control Explain the fundamentals and operation principles of Integral (I) Action and the disadvantages of proportional plus integral control Explain the fundamentals and operation principles of Derivative (D) Action in conjunction with P action Describe the operating principles of a PID Controller and explain the applications and advantages of PID control Describe Cascade, Forward, Split Range and Ratio Control operation principles Target Audience This course will benefit instrumentation, inspection, control, custody metering and process engineers and other technical staff. It is also suitable for piping engineers, pipelines engineers, mechanical engineers, operations engineers, maintenance engineers, plant/field supervisors and foremen and loss control coordinators. Trainer Your expert course leader is a Senior Mechanical & Instrumentation Engineer (UK, B. Sc., M.Eng., Ph D) with over 45 years of industrial experience in Process Control & Instrumentation, Pumps, Compressors, Turbines and Control Valve Technology. He is currently a Senior Independent Consultant to various petrochemical industries in the UK, USA, Oman, Kuwait and KSA where he provides consultancy services on both the application and operational constraints of process equipment in the oil & gas industries. During his early career, he held key positions in Rolls Royce (UK) where he was involved in the design of turbine blading for jet engines, subject to pre-specified distributions of pressure. During this period and since, he has also been closely involved in various aspects of Turbomachinery, Thermodynamics and Fluid Mechanics where he has become a recognised authority in these areas. Later, he joined the academic staff of University of Liverpool in the UK as a Professor in Mechanical Engineering Courses. A substantial part of his work has been concerned with detailed aspects of Flowmetering - both of single & multiphase flows. He has supervised doctoral research students in this area in collaboration with various European flowmeter manufacturers. He joined Haward Technology Middle East in 2002 and was later appointed as European Manager (a post which has since lapsed) and has delivered over 150 training courses in Flowmeasurement (single- and multi-phase), Control, Heat Exchangers, Pumps, Turbines, Compressors, Valve and Valve Selection as well as other topics throughout the UK, USA, Oman and Kuwait. During the last two years, he has delivered courses with other training companies operating in the Far and Middle East. He has published about 150 papers in various Engineering Journals and International Conferences and has contributed to textbooks on the topics listed above. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

LNG Fundamentals – Technical, Commercial, Standards and Safety Considerations in the LNG Industry Value Chain
By EnergyEdge - Training for a Sustainable Energy Future
Boost your knowledge in LNG fundamentals with our EnergyEdge course. Join our classroom training to gain valuable insights. Enroll now!

Artificial Lift and Real-Time Production Optimization in Digital Oilfield
By EnergyEdge - Training for a Sustainable Energy Future
About this training course Artificial lift systems are an important part of production operations for the entire lifecycle of an asset. Often, oil and gas wells require artificial lift for most of the life cycle. This 5-day training course offers a thorough treatment of artificial lift techniques including design and operation for production optimization. With the increasing need to optimize dynamic production in highly constrained cost environments, opportunities and issues related to real-time measurements and optimization techniques needs to be discussed and understood. Artificial lift selection and life cycle analysis are covered. These concepts are discussed and reinforced using case studies, quizzing tools, and exercises with software. Participants solve examples and class problems throughout the course. Animations and videos reinforce the concepts under discussion. Understanding of these important production concepts is a must have to exploit the existing assets profitably. Unique Features: Hands-on usage of SNAP Software to solve gas-lift exercises Discussion on digital oil field Machine learning applications in gas-lift optimization Training Objectives After the completion of this training course, participants will be able to: Understand the basics and advanced concepts of each form of artificial lift systems including application envelope, relative strengths, and weaknesses Easily recognize the different components from downhole to the surface and their basic structural and operational features Design and analyze different components using appropriate software tools Understand challenges facing artificial lift applications and the mitigation of these challenges during selection, design, and operation Learn about the role of digital oilfield tools and techniques and their applications in artificial lift and production optimization Learn about use cases of Machine learning and artificial intelligence in the artificial lift Target Audience This training course is suitable and will greatly benefit the following specific groups: Production, reservoir, completion, drilling and facilities engineers, analysts, and operators Anyone interested in learning about selection, design, analysis and optimum operation of artificial lift and related production systems will benefit from this course. Course Level Intermediate Advanced Training Methods The training instructor relies on a highly interactive training method to enhance the learning process. This method ensures that all participants gain a complete understanding of all the topics covered. The training environment is highly stimulating, challenging, and effective because the participants will learn by case studies which will allow them to apply the material taught in their own organization. Course Duration: 5 days in total (35 hours). Training Schedule 0830 - Registration 0900 - Start of training 1030 - Morning Break 1045 - Training recommences 1230 - Lunch Break 1330 - Training recommences 1515 - Evening break 1530 - Training recommences 1700 - End of Training The maximum number of participants allowed for this training course is 20. This course is also available through our Virtual Instructor Led Training (VILT) format. Prerequisites: Understanding of petroleum production concepts. Each participant needs a laptop/PC for solving class examples using software to be provided during class. Laptop/PC needs to have a current Windows operating system and at least 500 MB free disk space. Participants should have administrator rights to install software. Trainer Your expert course leader has over 35 years' work-experience in multiphase flow, artificial lift, real-time production optimization and software development/management. His current work is focused on a variety of use cases like failure prediction, virtual flow rate determination, wellhead integrity surveillance, corrosion, equipment maintenance, DTS/DAS interpretation. He has worked for national oil companies, majors, independents, and service providers globally. He has multiple patents and has delivered a multitude of industry presentations. Twice selected as an SPE distinguished lecturer, he also volunteers on SPE committees. He holds a Bachelor's and Master's in chemical engineering from the Gujarat University and IIT-Kanpur, India; and a Ph.D. in Petroleum Engineering from the University of Tulsa, USA. Highlighted Work Experience: At Weatherford, consulted with clients as well as directed teams on digital oilfield solutions including LOWIS - a solution that was underneath the production operations of Chevron and Occidental Petroleum across the globe. Worked with and consulted on equipment's like field controllers, VSDs, downhole permanent gauges, multiphase flow meters, fibre optics-based measurements. Shepherded an enterprise-class solution that is being deployed at a major oil and gas producer for production management including artificial lift optimization using real time data and deep-learning data analytics. Developed a workshop on digital oilfield approaches for production engineers. Patents: Principal inventor: 'Smarter Slug Flow Conditioning and Control' Co-inventor: 'Technique for Production Enhancement with Downhole Monitoring of Artificially Lifted Wells' Co-inventor: 'Wellbore real-time monitoring and analysis of fracture contribution' Worldwide Experience in Training / Seminar / Workshop Deliveries: Besides delivering several SPE webinars, ALRDC and SPE trainings globally, he has taught artificial lift at Texas Tech, Missouri S&T, Louisiana State, U of Southern California, and U of Houston. He has conducted seminars, bespoke trainings / workshops globally for practicing professionals: Companies: Basra Oil Company, ConocoPhillips, Chevron, EcoPetrol, Equinor, KOC, ONGC, LukOil, PDO, PDVSA, PEMEX, Petronas, Repsol, , Saudi Aramco, Shell, Sonatrech, QP, Tatneft, YPF, and others. Countries: USA, Algeria, Argentina, Bahrain, Brazil, Canada, China, Croatia, Congo, Ghana, India, Indonesia, Iraq, Kazakhstan, Kenya, Kuwait, Libya, Malaysia, Oman, Mexico, Norway, Qatar, Romania, Russia, Serbia, Saudi Arabia, S Korea, Tanzania, Thailand, Tunisia, Turkmenistan, UAE, Ukraine, Uzbekistan, Venezuela. Virtual training provided for PetroEdge, ALRDC, School of Mines, Repsol, UEP-Pakistan, and others since pandemic. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Methanol Bunkering & Fuel Cell Technology For Vessels
By EnergyEdge - Training for a Sustainable Energy Future
Enhance your knowledge with Energy Edge's course on methanol bunkering and fuel cell technology for vessels. Enroll now!

Data Analytics Workflows for Artificial Lift, Production and Facility Engineers
By EnergyEdge - Training for a Sustainable Energy Future
About this training course Business Impact: The main aim is to provide insight and understanding of data analytics and machine learning principles through applications. Field data is used to explain data-analysis workflows. Using easy to follow solution scripts, the participants will assess and extract value from the data sets. Hands-on solution approach will give them confidence to try out applicable techniques on data from their field assets. Data analysis means cleaning, inspecting, transforming, and modeling data with the goal of discovering new, useful information and supporting decision-making. In this hands-on 2-day training course, the participants learn some data analysis and data science techniques and workflows applied to petroleum production (specifically artificial lift) while reviewing code and practicing. The focus is on developing data-driven models while keeping our feet closer to the underlying oil and gas production principles. Unique Features: Eight business use cases covering their business impact, code walkthroughs for most all and solution approach. Industry data sets for participants to practice on and take home. No software or complicated Python frameworks required. Training Objectives After the completion of this training course, participants will be able to: Understand digital oil field transformation and its impact on business Examine machine learning methods Review workflows and code implementations After completing the course, participants will have a set of tools and some pathways to model and analyze their data in the cloud, find trends, and develop data-driven models Target Audience This training course is suitable and will greatly benefit the following specific groups: Artificial lift, production and facilities engineers and students to enhance their knowledge base, increase technology awareness, and improve the facility with different data analysis techniques applied on large data sets Course Level Intermediate Advanced Training Methods The course discusses several business use-cases that are amenable to data-driven workflows. For each use case, the instructor will show the solution using a data analysis technique with Python code deployed in the Google cloud. Trainees will solve a problem and tweak their solution. Course Duration: 2 days in total (14 hours). Training Schedule 0830 - Registration 0900 - Start of training 1030 - Morning Break 1045 - Training recommences 1230 - Lunch Break 1330 - Training recommences 1515 - Evening break 1530 - Training recommences 1700 - End of Training The maximum number of participants allowed for this training course is 20. This course is also available through our Virtual Instructor Led Training (VILT) format. Prerequisites: Understanding of petroleum production concepts Knowledge of Python is not a must but preferred to get the full benefit. The training will use the Google Collaboratory environment available in Google-Cloud for hands-on exercises Trainees will need to bring a computer with a Google Chrome browser and a Google email account (available for free) Trainer Your expert course leader has over 35 years' work-experience in multiphase flow, artificial lift, real-time production optimization and software development/management. His current work is focused on a variety of use cases like failure prediction, virtual flow rate determination, wellhead integrity surveillance, corrosion, equipment maintenance, DTS/DAS interpretation. He has worked for national oil companies, majors, independents, and service providers globally. He has multiple patents and has delivered a multitude of industry presentations. Twice selected as an SPE distinguished lecturer, he also volunteers on SPE committees. He holds a Bachelor's and Master's in chemical engineering from the Gujarat University and IIT-Kanpur, India; and a Ph.D. in Petroleum Engineering from the University of Tulsa, USA. Highlighted Work Experience: At Weatherford, consulted with clients as well as directed teams on digital oilfield solutions including LOWIS - a solution that was underneath the production operations of Chevron and Occidental Petroleum across the globe. Worked with and consulted on equipment's like field controllers, VSDs, downhole permanent gauges, multiphase flow meters, fibre optics-based measurements. Shepherded an enterprise-class solution that is being deployed at a major oil and gas producer for production management including artificial lift optimization using real time data and deep-learning data analytics. Developed a workshop on digital oilfield approaches for production engineers. Patents: Principal inventor: 'Smarter Slug Flow Conditioning and Control' Co-inventor: 'Technique for Production Enhancement with Downhole Monitoring of Artificially Lifted Wells' Co-inventor: 'Wellbore real-time monitoring and analysis of fracture contribution' Worldwide Experience in Training / Seminar / Workshop Deliveries: Besides delivering several SPE webinars, ALRDC and SPE trainings globally, he has taught artificial lift at Texas Tech, Missouri S&T, Louisiana State, U of Southern California, and U of Houston. He has conducted seminars, bespoke trainings / workshops globally for practicing professionals: Companies: Basra Oil Company, ConocoPhillips, Chevron, EcoPetrol, Equinor, KOC, ONGC, LukOil, PDO, PDVSA, PEMEX, Petronas, Repsol, , Saudi Aramco, Shell, Sonatrech, QP, Tatneft, YPF, and others. Countries: USA, Algeria, Argentina, Bahrain, Brazil, Canada, China, Croatia, Congo, Ghana, India, Indonesia, Iraq, Kazakhstan, Kenya, Kuwait, Libya, Malaysia, Oman, Mexico, Norway, Qatar, Romania, Russia, Serbia, Saudi Arabia, S Korea, Tanzania, Thailand, Tunisia, Turkmenistan, UAE, Ukraine, Uzbekistan, Venezuela. Virtual training provided for PetroEdge, ALRDC, School of Mines, Repsol, UEP-Pakistan, and others since pandemic. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Language Courses Abroad
By London Centre Of International Studies
Study abroad is about stepping out of your comfort zone, embracing a new culture and way of life, and coming home with unforgettable memories

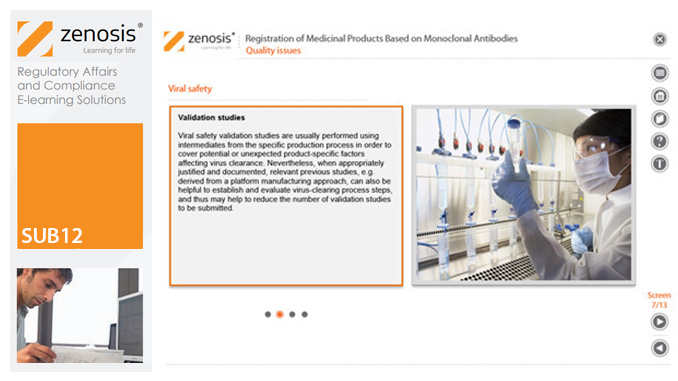
SUB12: Registration of Medicinal Products Based on Monoclonal Antibodies
By Zenosis
This module addresses characteristic issues influencing the registration of medicinal products based on monoclonal antibodies (mAbs), for use in humans. Regulatory requirements for the registration of biological medicinal products such as those based on mAbs differ in certain respects from those for small-molecule products. This is because of the distinct characteristics of biologics, such as complex structure and susceptibility to variation during manufacture.
Endometriosis Masterclass
By CCMIG
Endometriosis masterclass. Two day practical theory and hands on course on all aspects of endometriosis management. Expert faculty and live surgical cases.

Upstream Petroleum Economics, Risk and Fiscal Analysis
By EnergyEdge - Training for a Sustainable Energy Future
About this Training Course The 3-day hands-on petroleum economics training course provides a comprehensive overview of the practices of exploration and development petroleum economics and its application in valuing oil and gas assets to aid corporate decisions. Participants will gain a thorough understanding of the principles of economic analysis as well as practical instruction in analytical techniques used in the industry. The participants will learn how to construct economic models, to include basic fiscal terms, production and cost profiles and project timing. The resulting model will provide insights of how the various inputs affect value. Example exercises will be used throughout the course. Training Objectives Upon completion of this course, participants will be able to: Understand and construct petroleum industry cash flow projections Calculate, understand and know how to apply economic indicators Learn and apply risk analysis to exploration and production investments Evaluate and model fiscal/PSC terms of countries worldwide Target Audience The following oil & gas company personnel will benefit from the knowledge shared in this course: Geologists Explorationists Reservoir Engineers Project Accountants Contract Negotiators Financial Analysts New Venture Planners Economists Course Level Basic or Foundation Intermediate Trainer Your expert trainer has over 40 years' experience as a petroleum economist in the upstream oil and gas industry. He has presented over 230 oil and gas industry short courses worldwide on petroleum economics, risk, production sharing contracts (PSC) and fiscal analysis. In over 120 international oil industry consulting assignments, he has advised companies and governments in the Asia Pacific region on petroleum PSC and fiscal terms. He has prepared many independent valuations of petroleum properties and companies for acquisition and sale, as well as economics research reports on the oil and gas industry and including commercial support for oil field operations and investments worldwide. He has been involved in projects on petroleum royalties, design of petroleum fiscal terms, divestment of petroleum assets, and economic evaluation of assets and discoveries since the early 1990s to date. He has been working on training, consultancy, research and also advisory works in many countries including USA, UK, Denmark, Switzerland, Australia, New Zealand, Indonesia, India, Iran, Malaysia, Thailand, Vietnam, Brunei, Egypt, Libya, and South Africa. POST TRAINING COACHING SUPPORT (OPTIONAL) To further optimise your learning experience from our courses, we also offer individualized 'One to One' coaching support for 2 hours post training. We can help improve your competence in your chosen area of interest, based on your learning needs and available hours. This is a great opportunity to improve your capability and confidence in a particular area of expertise. It will be delivered over a secure video conference call by one of our senior trainers. They will work with you to create a tailor-made coaching program that will help you achieve your goals faster. Request for further information post training support and fees applicable Accreditions And Affliations

Search By Location
- usa Courses in London
- usa Courses in Birmingham
- usa Courses in Glasgow
- usa Courses in Liverpool
- usa Courses in Bristol
- usa Courses in Manchester
- usa Courses in Sheffield
- usa Courses in Leeds
- usa Courses in Edinburgh
- usa Courses in Leicester
- usa Courses in Coventry
- usa Courses in Bradford
- usa Courses in Cardiff
- usa Courses in Belfast
- usa Courses in Nottingham