- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
22698 Courses
Report Writing 1 Day Training in Peterborough
By Mangates
Report Writing 1 Day Training in Peterborough

Report Writing 1 Day Training in Slough
By Mangates
Report Writing 1 Day Training in Slough

Report Writing 1 Day Training in Maidstone
By Mangates
Report Writing 1 Day Training in Maidstone

Report Writing 1 Day Training in Liverpool
By Mangates
Report Writing 1 Day Training in Liverpool

Report Writing 1 Day Training in Newcastle
By Mangates
Report Writing 1 Day Training in Newcastle

The “ISO 20387 Lead Assessor Course” is a comprehensive program designed to equip individuals with the knowledge and skills needed to assess and evaluate biobanking systems in accordance with ISO 20387:2018. This course focuses on the principles of assessment, audit methodologies, and the specific requirements of ISO 20387. Participants will learn how to lead and conduct assessments of biobanking facilities and organizations to ensure compliance with the standard.

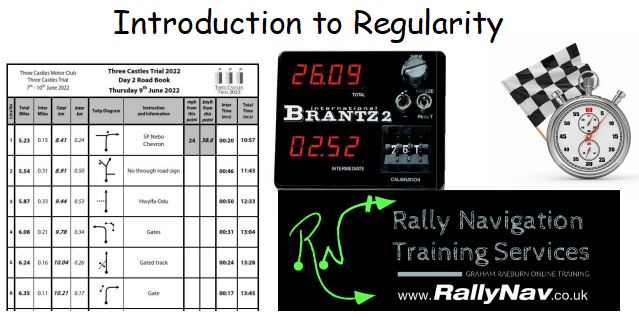
Rally Navigation - Introduction to Regularity Timing
By Rally Navigation Training Services
Historic Road Rallying training webinar on Regularity focusing on the Jogularity and Cumulative Speed Table styles.
Learn to craft top-notch videos for your business using your mobile phone in this interactive workshop. Enhance marketing, visibility, and attract more clients through video. All-in-one workshop for diving into the world of business videos. #VideoCreation #BusinessBoost

Search By Location
- EQ Courses in London
- EQ Courses in Birmingham
- EQ Courses in Glasgow
- EQ Courses in Liverpool
- EQ Courses in Bristol
- EQ Courses in Manchester
- EQ Courses in Sheffield
- EQ Courses in Leeds
- EQ Courses in Edinburgh
- EQ Courses in Leicester
- EQ Courses in Coventry
- EQ Courses in Bradford
- EQ Courses in Cardiff
- EQ Courses in Belfast
- EQ Courses in Nottingham

