- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
169 Courses
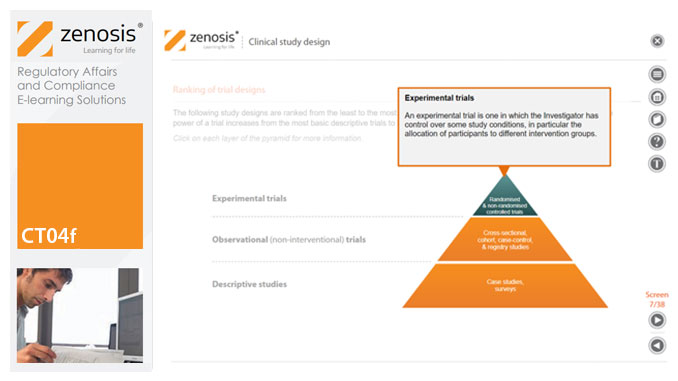
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
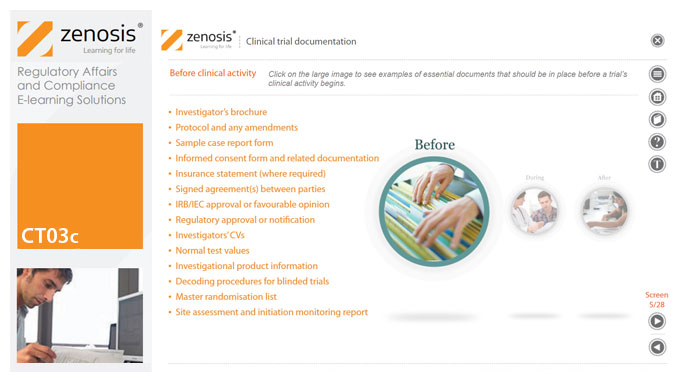
CT03c - Clinical trial documentation
By Zenosis
Regulatory authorities tend to abide by the maxim that ‘If it isn’t documented, it didn’t happen’. Rigorous documentation of all aspects of a clinical trial is necessary to provide evidence of GCP and compliance with regulatory requirements, as well as enabling effective management of the trial. In this short course we describe important examples of the documents designated by ICH GCP as essential to the conduct of a clinical trial.
Preparing for a Job Interview-CPD Approved
By BAB Business Group
Interviews are two-way processes, where the employer and the interviewee can both decide if they are a good fit. This short course will provide you with some useful tips for how to prepare for an interview including preparing answers to common questions. The course also covers other things to consider in advance of the interview including planning how you will get there. It briefly covers some of the key differences between online and face to face interviews.

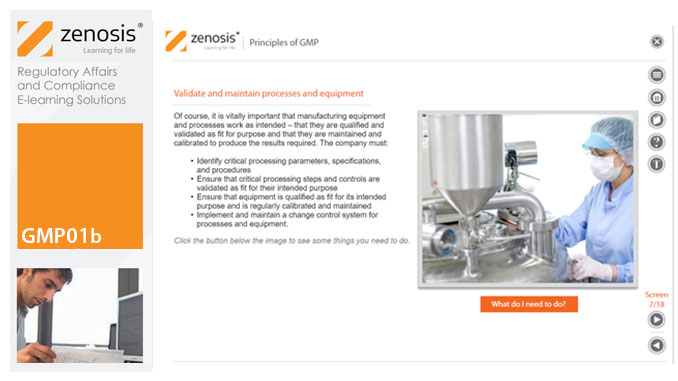
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
CT04b - Clinical protocol design
By Zenosis
Clinical trial protocols are an essential part of clinical trial design. Protocol documents are critical to conducting safe and cost-effective investigations. Protocol documents are large and complex, containing comprehensive information relating to purpose, design and conduct of a clinical trial. Aspects of a protocol include patient eligibility criteria, and treatment specifications. This short course provides an overview of clinical trial protocols. Opportunities to improve a clinical trial protocol for regulatory approval are also discussed.
Video Editing for Beginners Short Course Mini Bundle
By Compete High
The Video Editing for Beginners Short Course Mini Bundle is your entry into the world of digital visuals—minus the headache of over-complicated tech talk. You’ll explore Adobe Premiere Pro, video planning, drawing fundamentals, basic animation, and time management (because editing takes longer than you'd expect). If you’ve ever tried to cut a 30-minute video into something that doesn’t bore people by minute two, you already know the value of knowing your tools. This course keeps it structured, simple, and creative—perfect for people ready to edit without overthinking the timeline. Learning Outcomes: Edit and cut videos using Adobe Premiere Pro software. Understand the basics of animation and motion graphics. Plan and organise ideas for better video development flow. Apply drawing concepts for layout or visual storytelling. Improve time management when working on creative projects. Use software tools effectively for beginner video editing. Who Is This Course For: Beginners exploring video editing for creative or casual use. Content creators looking to edit their own footage confidently. Social media users making engaging videos and reels. Freelancers offering editing alongside other creative services. Bloggers and vloggers wanting more polished video content. Students learning creative tools outside of formal environments. Professionals creating videos for business or team updates. Anyone tired of using ten apps to crop one clip. Career Path: Junior Video Editor – £26,000/year Content Creator – £27,000/year Social Media Video Assistant – £25,000/year Animation Intern – £23,000/year Marketing Assistant (Video Focus) – £28,000/year Freelance Editor (Entry-Level) – £24,000/year

CT04c - Clinical trial preparation
By Zenosis
The demands on quality from clinical trials are increasing. Quantitative aspects of clinical trials, such as the mass of study data to be collected, the multiple investigational sites, and the need to meet predetermined timelines, often supersede qualitative features. Therefore, addressing basic requirements for quality management is essential when preparing a clinical trial. This short course describes the core elements required for the establishment of a clinical trial and provides an overview of the role of the sponsor in supporting and improving trial quality.
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
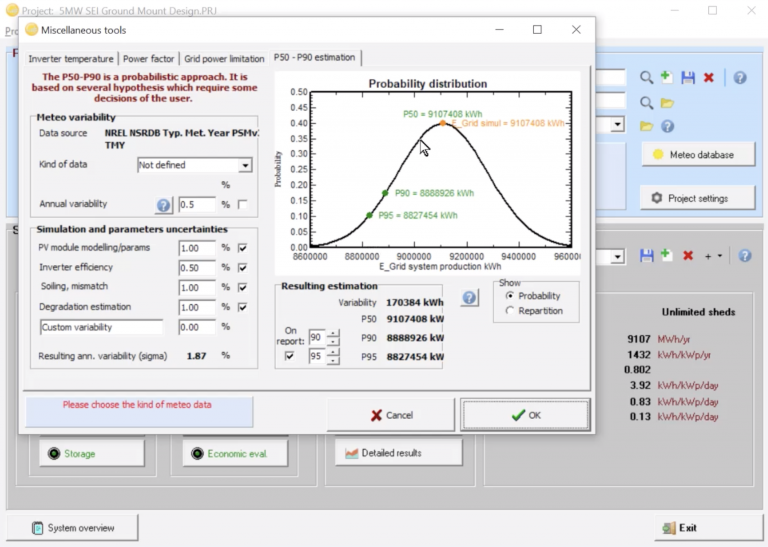
CE524: PVsyst for PV System Production Modeling
By Solar Energy International (SEI)
This short course is targeted towards beginning users, and will show you in detail how to get started creating accurate production estimates for any size PV system, from residential to large-scale. Learn how to find and import the correct meteorological data, create system variants for any size system, and accurately define the orientation, shading scene, and detailed system losses. By the end of this course you will be confidently simulating production and printing reports to share.
Search By Location
- short course Courses in London
- short course Courses in Birmingham
- short course Courses in Glasgow
- short course Courses in Liverpool
- short course Courses in Bristol
- short course Courses in Manchester
- short course Courses in Sheffield
- short course Courses in Leeds
- short course Courses in Edinburgh
- short course Courses in Leicester
- short course Courses in Coventry
- short course Courses in Bradford
- short course Courses in Cardiff
- short course Courses in Belfast
- short course Courses in Nottingham