- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
147 Courses
ISO 27001 (2022) Lead Implementer
By Training Centre
The IECB ISO/IEC 27001 Lead Implementer training enables you to develop the necessary expertise to support an organization in establishing, implementing, managing and maintaining an Information Security Management System (ISMS) based on ISO 27001 During this training course, you will also gain a thorough understanding of the best practices of Information Security Management Systems to secure the organization`s sensitive information and improve the overall performance and effectiveness. About This Course After mastering all the necessary concepts of Information Security Management Systems, you can sit for the exam and gain the 'IECB Certified ISO/IEC 27001 Lead Implementer' credential. By holding an IECB Lead Implementer Certificate, you will be able to demonstrate that you have the practical knowledge and professional capabilities to implement ISO/IEC 27001 in an organization. This official IECB course is delivered in either our Live Online or Classroom format, as follows; Day 1: Introduction to ISO/IEC 27001 and initiation of an ISMS Day 2: Plan the implementation of an ISMS Day 3: Implementation of an ISMS Day 4: ISMS monitoring, measurement, continuous improvement and preparation for a certification audit, as well as the examination. Learning Objectives Acknowledge the correlation between ISO/IEC 27001, ISO 27002 and other standards and regulatory frameworks Master the concepts, approaches, methods and techniques used for the implementation and effective management of an ISMS Learn how to interpret the ISO/IEC 27001 requirements in the specific context of an organization Learn how to support an organization to effectively plan, implement, manage, monitor and maintain an ISMS Acquire the expertise to advise an organization in implementing Information Security Management System best practices The exam covers the following competency domains: Domain 1: Fundamental principles and concepts of an Information Security Management System (ISMS) Domain 2: Information Security Management System controls and best practices based on ISO/IEC 27002 Domain 3: Planning an ISMS implementation based on ISO/IEC 27001 Domain 4: Implementing an ISMS based on ISO/IEC 27001 Domain 5: Performance evaluation, monitoring and measurement of an ISMS based on ISO/IEC 27001 Domain 6: Continual improvement of an ISMS based on ISO/IEC 27001 Domain 7: Preparing for an ISMS certification audit Prerequisites A foundational understanding of ISO/IEC 27001 and knowledge of implementation principles. What's Included? Refreshments & Lunch (Classroom only) Course Slide Deck Official Study Materials CPD Certificate The Exam Who Should Attend? The course is suitable for any of the following roles; Security Analyst Network Administrator Data Architect Security Manager Our Guarantee We are an approved IECB Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training and exam retake offered Accreditation Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 240 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Provided by This course is Accredited by NACS and Administered by theIECB.

Level 7 NVQ Diploma in Construction Senior Management
By Dynamic Training and Assessments Ltd
Level 7 NVQ Diploma in Construction Senior Management

ISO 13485 Lead Implementer
By Training Centre
During this training course, you will also gain a thorough understanding of the best practices of Medical Devices Quality Management Systems and be able to improve an organization`s overall performance by consistently providing safe and qualitative medical devices. After mastering all the necessary concepts of Medical Devices Quality Management Systems, you can sit for the exam and gain the "Certified ISO 13485 Lead Implementer' Certificate. By holding this Certificate, you will be able to demonstrate that you have the practical knowledge and professional capabilities to implement ISO 13485 in an organization. About This Course Learning Objectives Acknowledge the correlation between ISO 13485 and other standards and regulatory frameworks Master the concepts, approaches, methods and techniques used for the implementation and effective management of a MDQMS Learn how to interpret the ISO 13485 requirements in the specific context of an organization Learn how to support an organization to effectively plan, implement, manage, monitor and maintain a MDQMS Acquire the expertise to advise an organization in implementing Medical Devices Quality Management System best practices Course Agenda Day 1: Introduction to ISO 13485 and initiation of a MDQMS Day 2: Plan the implementation of a MDQMS Day 3: Implementation of a MDQMS Day 4: MDQMS monitoring, measurement, continuous improvement and preparation for a certification audit, and the final exam. Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Prerequisites A fundamental understanding of ISO 13485 and comprehensive knowledge of implementation principles. What's Included? Certification fees are included on the exam price Training material containing over 450 pages of information and practical examples will be distributed An attestation of course completion worth 32 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course. In case of exam failure, you can retake the exam within 12 months for free Who Should Attend? Managers or consultants involved in Medical Devices Quality Management Expert advisors seeking to master the implementation of a Medical Devices Quality Management System Individuals responsible for maintaining conformance with MDQMS requirements MDQMS team members Accreditation Provided by This course is Accredited by NACS and Administered by the IECB

ISO 27032: 2023 Lead Cybersecurity Manager
By Training Centre
ISO/IEC 27032: 2023 Lead Cybersecurity Manager training enables you to acquire the expertise and competence needed to support an organization in implementing and managing a Cybersecurity program based on ISO 27032: 2023 and the NIST Cybersecurity framework. About This Course During this training course, you will gain a comprehensive knowledge of Cybersecurity, the relationship between Cybersecurity and other types of IT security, and stakeholders' role in Cybersecurity. After mastering all the necessary concepts of Cybersecurity, you can sit for the exam and gain "Certified ISO/IEC 27032 Lead Cybersecurity Manager' Certification. By holding this certification, you will be able to demonstrate that you have the practical knowledge and professional capabilities to support and lead a team in managing Cybersecurity. Learning objectives Acquire comprehensive knowledge on the elements and operations of a Cybersecurity Program in conformance with ISO/IEC 27032 and NIST Cybersecurity framework Acknowledge the correlation between ISO 27032, NIST Cybersecurity framework and other standards and operating frameworks Master the concepts, approaches, standards, methods and techniques used to effectively set up, implement, and manage a Cybersecurity program within an organization Learn how to interpret the guidelines of ISO/IEC 27032 in the specific context of an organization Master the necessary expertise to plan, implement, manage, control and maintain a Cybersecurity Program as specified in ISO/IEC 27032 and NIST Cybersecurity framework Acquire the necessary expertise to advise an organization on the best practices for managing Cybersecurity Educational approach This training is based on both theory and best practices used in the implementation and management of a Cybersecurity Program Lecture sessions are illustrated with examples based on case studies Practical exercises are based on a case study which includes role playing and discussions Practical tests are similar to the Certification Exam Prerequisites A fundamental understanding of ISO/IEC 27032: 2023 and comprehensive knowledge of Cybersecurity. What's Included? Refreshments & Lunch (Classroom courses only) Course Slide Deck Official Study Guides CPD Certificate The Exam Who Should Attend? Cybersecurity professionals Information Security experts Professionals seeking to manage a Cybersecurity program Individuals responsible to develop a Cybersecurity program IT specialists Information Technology expert advisors IT professionals looking to enhance their technical skills and knowledge Accreditation Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Provided by This course is Accredited by NACS and Administered by the IECB

ISO 22301 Lead Implementer
By Training Centre
Delivered in either our Live Online (4 days) or in a Classroom environment (5 days), the ISO 22301 Lead Implementer training course enables you to develop the necessary expertise to support an organization in establishing, implementing, managing and maintaining a Business Continuity Management System (BCMS) based on ISO 22301. About This Course During this training course, you will gain a thorough understanding of the best practices of Business Continuity Management Systems and be able to provide a framework that allows the organization to continue operating efficiently during disruptive events. After mastering all the necessary concepts of Business Continuity Management Systems, you can sit for the exam and gain the "ISO 22301 Lead Implementer' credential. By holding this Certificate, you will demonstrate that you have the practical knowledge and professional capabilities to implement ISO 22301 in an organization. Learning objectives Acknowledge the correlation between ISO 22301 and other standards and regulatory frameworks Master the concepts, approaches, methods and techniques used for the implementation and effective management of a BCMS Learn how to interpret the ISO 22301 requirements in the specific context of an organization Learn how to support an organization to effectively plan, implement, manage and maintain a BCMS Acquire the expertise to advise an organization in implementing Business Continuity Management System best practices Educational approach This training is based on both theory and best practices used in the implementation of a BCMS Lecture sessions are illustrated with examples based on case studies Practical exercises are based on a case study which includes role playing and discussions Practice tests are similar to the Certification Exam Prerequisites A foundational understanding of ISO 22301 and knowledge of implementation principles. What's Included? Refreshments & Lunch (Classroom based only) Course Slide Deck Official Study Materials CPD Certificate The Exam fees Who Should Attend? Managers or consultants involved in Business Continuity Management Expert advisors seeking to master the implementation of a Business Continuity Management System Individuals responsible for maintaining conformance with BCMS requirements BCMS team members Accreditation Our Guarantee We are an approved IECB Training Partner. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and only pay for the exam. Assessment The exam is a 12 question essay type. The pass mark for the exam is 70% and should be completed within the 150 minutes allocated. Results are provided within 24 hours of completion. Provided by This course is Accredited by NACS and Administered by the IECB

ISO 37301 Lead Implementer
By Training Centre
A CMS provides organizations a structured approach to meet all compliance obligations, i.e., requirements that they mandatorily have to comply with such as laws, regulations, court rulings, permits, licenses, as well as those that they voluntarily choose to comply with such as internal policies and procedures, codes of conduct, standards, and agreements with communities or NGOs. About This Course The benefits of implementing a compliance management system (CMS) based on ISO 37301 are manifold: helping the organization avoid or mitigate the costs, risks, and damage of noncompliance, ensuring the long-term sustainability of the organization, promoting trust and confidence, encouraging good governance practices, due diligence, and ethically sound business dealings, etc. The ISO 37301 Lead Implementer training course provides the knowledge needed to establish, implement, manage, maintain, and continually improve a CMS. It aims to provide an in-depth understanding of ISO 37301 requirements, as well as the best practices and approaches used for the implementation and subsequent maintenance of the compliance management system. The training course enables you to help organizations establish processes needed to adhere to all compliance obligations and establish controls that proactively prevent noncompliance and contribute to the creation of a culture of integrity, transparency, and openness. The training course is followed by the certification exam. If you pass, you gain the 'Certified ISO 37301 Lead Implementer' credential. This credential validates your professional capabilities and competences to implement a CMS in an organization based on the requirements of ISO 37301. This training course will help you: Gain a comprehensive understanding of the concepts, approaches, methods, and techniques used for the implementation and effective management of a CMS Acknowledge the correlation between ISO 37301 and other standards and regulatory frameworks Gain the ability to interpret the requirements of ISO 37301 in the specific context of an organization Develop the necessary knowledge and expertise to support an organization in effectively planning, implementing, managing, monitoring, and maintaining a CMS Acquire the expertise to advise an organization in implementing CMS best practices Assessment Delegates sit a combined exam, consisting of in-course quizzes and exercises, as well as a final 12 question, essay type exam on Day 4 of the course. The overall passing score is 70%, to be achieved within the 150 minute time allowance. Exam results are provided within 24 hours, with both a Certificate and a digital badge provided as proof of success. Prerequisites The main requirements for participating in this training course are a basic knowledge of ISO management system standards, as well as a general understanding of ISO 37301 (or ISO 19600 guidelines) and the MS implementation principles. What's Included? Certification fees are included in the exam price. Training material of over 450 pages of information and practical examples will be provided. An attestation of course completion worth 31 CPD (Continuing Professional Development) credits will be issued to participants who have attended the training course. In case of exam failure, candidates can retake the exam once for free within 12 months following the initial exam date. Who Should Attend? Managers, consultants, and compliance officers wishing to develop a thorough understanding of ISO 37301 requirements for a compliance management system Managers and consultants seeking a comprehensive CMS implementation framework Compliance officers responsible for practicing due diligence with regard to compliance risks Individuals wishing to contribute in maintaining organizational integrity by supporting ethical behaviour Managers and members of governance, risk management, and compliance teams Individuals aspiring to become compliance officers or compliance management consultant Accreditation Provided by This course is Accredited by NACS and Administered by the IECB

Lead Cloud Security Manager
By Training Centre
This training course is designed to help participants acquire the knowledge and skills needed to support an organization in effectively planning, implementing, managing, monitoring, and maintaining a cloud security program based on ISO/IEC 27017 and ISO/IEC 27018. It provides a comprehensive elaboration of cloud computing concepts and principles, cloud computing security risk management, cloud-specific controls, cloud security incident management, and cloud security testing. About This Course Learning objectives Gain a comprehensive understanding of the concepts, approaches, methods, and techniques used for the implementation and effective management of a cloud security program Acknowledge the correlation between ISO/IEC 27017, ISO/IEC 27018, and other standards and regulatory frameworks Gain the ability to interpret the guidelines of ISO/IEC 27017 and ISO/IEC 27018 in the specific context of an organization Develop the necessary knowledge and competence to support an organization in effectively planning, implementing, managing, monitoring, and maintaining a cloud security program Acquire the practical knowledge to advise an organization in managing a cloud security program by following best practices Course Agenda Day 1: Introduction to ISO/IEC 27017 and ISO/IEC 27018 and the initiation of a cloud security program Day 2: Cloud computing security risk management and cloud-specific controls Day 3: Documented information management and cloud security awareness and training Day 4: Cloud security incident management, testing, monitoring, and continual improvement; the examination Additional Information Certification fees are included in the exam price. An attendance record worth 31 CPD (Continuing Professional Development) credits will be issued to the participants who have attended the training course. In case candidates fail the exam, they can retake it within 12 months of the initial attempt for free. Accreditation Prerequisites The main requirement for participating in this training course is having a fundamental understanding of ISO/IEC 27017 and ISO/IEC 27018 and a general knowledge of cloud computing concepts. Who Should Attend? Cloud security and information security professionals seeking to manage a cloud security program Managers or consultants seeking to master cloud security best practices Individuals responsible for maintaining and managing a cloud security program Technical experts seeking to enhance their cloud security knowledge Cloud security expert advisors What's Included? Delegates will be provided with; Course Slide deck Participant Guide Exam fees Our Guarantee We are an Accredited Training Provider of IECB. You can learn wherever and whenever you want with our robust classroom and interactive online training courses. Our courses are taught by qualified practitioners with a minimum of 25 years commercial experience. We strive to give our delegates the hands-on experience. Our courses are all-inclusive with no hidden extras. The one-off cost covers the training, all course materials, and exam voucher. Our aim: To achieve a 100% first time pass rate on all our instructor-led courses. Our Promise: Pass first time or 'train' again for FREE. *FREE training offered for retakes - come back within a year and only pay for the exam. Assessment The Certified Lead Cloud Security Manager exam meets the requirements of the National Accreditation Service's Examination and Certification Program (ECP). It covers the following competency domains: Domain 1: Fundamental principles and concepts of cloud computing Domain 2: Information security policy for cloud computing and documented information management Domain 3: Cloud computing security risk management Domain 4: Cloud-specific controls based on ISO/IEC 27017 and ISO/IEC 27018 and best practices Domain 5: Cloud security awareness, training, roles, and responsibilities Domain 6: Cloud security incident management Domain 7: Cloud security testing, monitoring, and continual improvement All delegates attending an official training course will be offered the opportunity to sit the associated examination. To pass the examination, a passing score of 70% must be obtained by answering 12 essay type questions covering the scope of the course materials. Successful examination candidates will be issued with a Certificate confirming a passing grade along with the relevant CPD certificate. Provided by This course is Accredited by NACS and Administered by the IECB

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

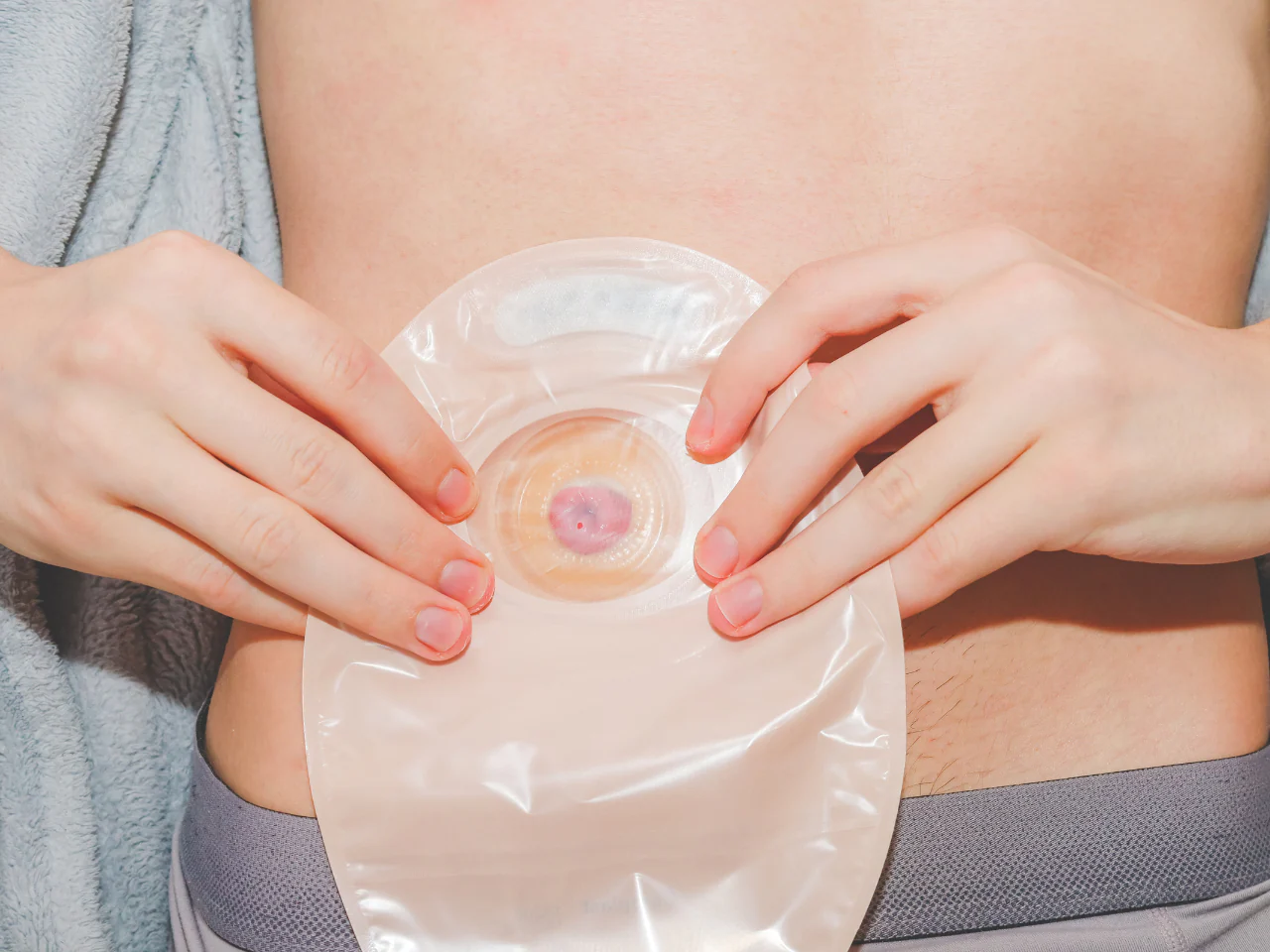
An Understanding of Stoma Care
By Guardian Angels Training
Gain essential knowledge and practical skills to provide effective care for patients with stomas with our "An Understanding of Stoma Care" course. Learn how to maintain stoma health and support patients through their journey.
Diploma in Business Continuity & Resiliency Management
By BCMcourses.com
This is our new Diploma course in Business Continuity & Resiliency Management. It includes the BCRP (Business Continuity and Resiliency Professional) exam and designation for free ($ 500 value). This course provides an intensive, hands-on workshop experience covering all major aspects for the development, implementation and ongoing management of an effective Business Continuity Plan for corporations, any size of business, healthcare, NGO and government organizations. It is equivalent to our 5-day in-person 'Diploma in BCRP' course. It utilizes a 21st century skills curriculum to enable you to achieve the knowledge and expertise required to succeed in your personal involvement in Business Continuity Planning and within your work environment. The course integrates “Best Principles” for Business Continuity Planning with “Best Practices”, using a case study, real-life scenarios and examples to deepen your understanding. This course is accredited by the National Institute for Business Continuity Management (https://www.NIBCM.net) and leads to their professional designation of Business Continuity & Resiliency Professional (BCRP), as well as our Diploma in Business Continuity and Resiliency Management. In addition, the course provides you with real-life tools for Business Continuity Planning. It follows a case study approach, and engages you in solving meaningful problems with your own organization. The course also provides asynchronous interaction with the course instructor for assignments and any questions that may arise.

Search By Location
- Management of Asthma Courses in London
- Management of Asthma Courses in Birmingham
- Management of Asthma Courses in Glasgow
- Management of Asthma Courses in Liverpool
- Management of Asthma Courses in Bristol
- Management of Asthma Courses in Manchester
- Management of Asthma Courses in Sheffield
- Management of Asthma Courses in Leeds
- Management of Asthma Courses in Edinburgh
- Management of Asthma Courses in Leicester
- Management of Asthma Courses in Coventry
- Management of Asthma Courses in Bradford
- Management of Asthma Courses in Cardiff
- Management of Asthma Courses in Belfast
- Management of Asthma Courses in Nottingham