- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
27924 Courses
Level 5 QLS Diploma in Construction Site Management & Cost Estimation
By Imperial Academy
Optimize construction projects with efficient site management and accurate cost estimation for success in the UK market

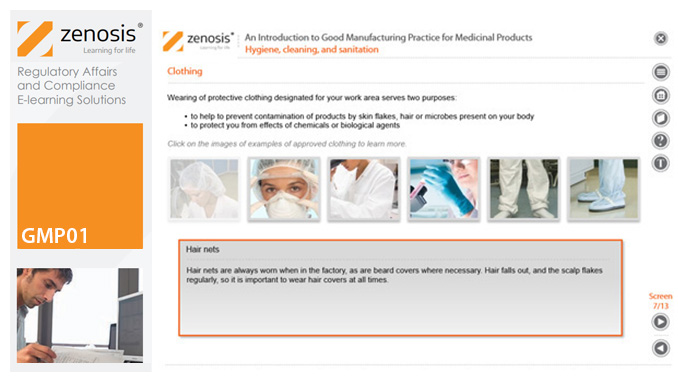
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
**Be prepared for the upcoming Hiring Season by enhancing your professional skillsets with Apex Learning! Get Hard Copy + PDF Certificate + Transcript + Student ID Card as a Gift - Enrol Now** Tired of browsing and searching for the course you are looking for? Can't find the complete package that fulfils all your needs? Then don't worry as you have just found the solution. Take a minute and look through this 14-in-1 extensive bundle that has everything you need to succeed in Financial Advisor and other relevant fields! After surveying thousands of learners just like you and considering their valuable feedback, this all in one Financial Advisor bundle has been designed by industry experts. We prioritised what learners were looking for in a complete package and developed this in-demand Financial Advisor course that will enhance your skills and prepare you for the competitive job market. Also, our Financial Advisor experts are available for answering your queries and help you along your learning journey. Advanced audiovisual learning modules of these courses are broken down into little chunks so that you can learn at your own pace without being overwhelmed by too much material at once. Furthermore, to help you showcase your expertise in Financial Advisor, we have prepared a special gift of 1 hardcopy certificate and 1 PDF certificate for the title course completely free of cost. These certificates will enhance your credibility and encourage possible employers to pick you over the rest. This Financial Advisor Bundle Consists of the following Premium courses: Course 01: Financial Advisor Course 02: Financial Management Course 03: Investment Course 04: Capital Budgeting & Investment Decision Rules Course 05: Budgeting and Forecasting Course 06: Level 3 Tax Accounting Course 07: Team Management Course 08: Intermediate Economics Level 3 Course 09: Internal Audit Training Diploma Course 10: Forex Trading Level 3 Course 11: Stock Market Investing for Beginners Course 12: Fraud Management & Anti Money Laundering Awareness Complete Diploma Course 13: Commercial Law 2021 Course 14: Level 2 Microsoft Office Essentials Benefits you'll get choosing Apex Learning: One payment, but lifetime access to 14 CPD courses Certificates, student ID for the title course included in a one-time fee Full tutor support available from Monday to Friday Free up your time - don't waste time and money travelling for classes Accessible, informative modules taught by expert instructors Learn at your ease - anytime, from anywhere Study the course from your computer, tablet or mobile device CPD accredited course - improve the chance of gaining professional skills How will I get my Certificate? After successfully completing the course you will be able to order your CPD Accredited Certificates (PDF + Hard Copy) as proof of your achievement. PDF Certificate: Free (For The Title Course) Hard Copy Certificate: Free (For The Title Course) Curriculum of Bundle Course 01: Financial Advisor Introduction to Finance Essential Skill Financial Planning Financial Risk Management and Assessment Investment Planning Divorce Planning Google Analytics and many more.... Course 02: Financial Management Introduction to Financial Management Fundamentals of Budgeting The Balance Sheet The Income Statement The Cash Flow Statement Statement of Stockholders' Equity Analysing and Interpreting Financial Statements International Aspects of Financial Management and many more.... Course 03: Investment Introduction to Investment Types and Techniques of Investment Key Concepts in Investment Understanding the Finance Investing in Bond Market Investing in Stock Market Risk and Portfolio Management and many more.... Course 04: Capital Budgeting & Investment Decision Rules Introduction NPV Method Payback Period Method Internal Rate of Return (IRR) Evaluating Projects in Different Lives Conclusion and many more.... Course 05: Budgeting and Forecasting Introduction Why Budget and Forecasts Is budget planning a paper exercise Operational and Financial Budget Detail Budget Requirement Components - Revenue Budgets Components - Cost Budget Qualitative Aspects Process of Making Budget Process of Budgeting - Logical Steps Example we used in Class to demonstrate a broad process in budget and planning Cost Budget Process - Other aspects and many more.... Course 06: Level 3 Tax Accounting Tax System and Administration in the UK Tax on Individuals National Insurance Fundamentals of Income Tax Payee, Payroll and Wages Value Added Tax Corporation Tax Double Entry Accounting Management Accounting and Financial Analysis Career as a Tax Accountant in the UK and many more.... Course 07: Presenting Financial Information Presenting Financial Information The Hierarchy of Performance Indicators The Principle of Effective Reports Methods of Presenting Performance Data The Pareto Chart: Highlighting Priorities Exercise: The Control Chart An Example Management Report Interpreting Performance Data Supporting Colleagues by Giving Feedback Data Visualisation Part 01, Part 02, Part 03, Part 04 and many more.... Course 08: Intermediate Economics Level 3 An Introduction to Economics The Market System and the Circular Flow Model Supply, Demand and Prices Prices Elasticity Market Failures Production and Costs Money, Banking and the Financial System Measuring GDP and Economic Growth Unemployment Inflation Income Distribution and Poverty International Finance Fiscal Policy and many more.... Course 09: Internal Audit Training Diploma Auditing as a Form of Assurance Internal Audit Procedures Technology-based Internal Audit Audit Interviews Reporting Audit Outcome UK Internal Audit Standards Career as an Auditor and many more.... Course 10: Forex Trading Level 3 Introduction to Forex Trading Kinds of Foreign Exchange Market Money Management Fundamental Analysis Technical Analysis Pitfalls and Risks Managing Risk and many more.... Course 11: Stock Market Investing for Beginners Module 01: Introduction to the Course Module 02: Introduction to Stocks Module 03: Money Required for Primary Investment Module 04: Opening an Investment Account Module 05: Brokerage Account Walkthrough Module 06: Finding Winning Stocks Module 07: Earning from Dividends Module 08: Diversifying Portfolio Module 09: Investment Plan Module 10: Rebalancing Portfolio Module 11: Understanding Order Types Module 12: Investment Tax Module 13: Investment Rules: Rule-1, 2, 3, 4, 5 Module 14: Stock Market Dictionary Module 15: Setting Up the Trading Platform Course 12: Fraud Management & Anti Money Laundering Awareness Complete Diploma Introduction to Money Laundering Proceeds of Crime Act 2002 Risk-based Approach Customer Due Diligence Record Keeping Suspicious Conduct and Transactions Awareness and Training and many more.... Course 13: Commercial Law 2021 Introduction of Commercial law Sales of Goods Law Consumer Law and Protection E-Commerce Law Competition Law and many more.... Course 14: Level 2 Microsoft Office Essentials Excel 2016 Getting Started with Microsoft Office Excel 2016 Performing Calculations Modifying a Worksheet Printing Workbooks Managing Workbooks Word 2016 Getting Started with Word Working More Efficiently Managing Lists Inserting Graphic Objects Controlling Page Appearance Preparing to Publish a Document Workbooks - Microsoft Word 2016 (Beginner) PowerPoint 2016 Presentation Basics Inserting Options Working with Objects Access 2016 Introduction to Access Access Forms Working with Reports and many more.... CPD 145 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone from any background can enrol in this Financial Advisor bundle. Requirements Our Financial Advisor course is fully compatible with PCs, Macs, laptops, tablets and Smartphone devices. Career path Having this Financial Advisor expertise will increase the value of your CV and open you up to multiple job sectors. Certificates Certificate of completion Digital certificate - Included You will get the PDF Certificate for the title course (Financial Advisor) absolutely Free! Certificate of completion Hard copy certificate - Included You will get the Hard Copy certificate for the title course (Financial Advisor) absolutely Free! Other Hard Copy certificates are available for £10 each. Please Note: The delivery charge inside the UK is £3.99, and the international students must pay a £9.99 shipping cost.

Description Complementary Medicine Diploma Complementary Medicine is a broad domain of numerous diagnostic and therapeutic disciplines that are used in conjunction with mainstream medical care. Complementary medicine generally exists beyond the scope of medical institutions where traditional healthcare is taught and provided. Complementary medicine encompasses various health systems, modalities and practices along with their philosophies and theories. The practices and beliefs defined by the users of such health systems on preventing and curing diseases are also a part of complementary medicine. Complementary medicine is often referred to as complementary therapies. Complementary medicine includes popular health systems like Acupressure, Acupuncture, Reflexology, Aromatherapy, Homeopathy, Ayurveda and Yoga. The term complementary medicine is often considered synonymous with 'Complementary and Alternative Medicine. Nonetheless, a distinction can be made between the two. Complementary medicine can be seen as healthcare practices used along with conventional healthcare whereas alternative medicine acts as a substitute for conventional healthcare. Complementary medicine has maintained its popularity despite being considered as unconventional medicine and the lack of adequate scientific evidence of its working. The reason is that many people have had powerful healing experiences that they claim cannot be denied just because of the lack of notable support from the scientific community. Complementary medicine is also believed to lessen a patient's discomfort during the course of traditional treatment. For instance, acupuncture has proven to reduce a patient's discomfort after surgery.Complementary Medicine Diploma is an introductory course in complementary medicine that covers a huge range of conventional therapies with essential information on each therapy. Complementary Medicine Diploma discusses what each complementary therapy is about, how it works, what it offers and what evidence supports it. After completing Complementary Medicine Diploma, you will be well-informed and up-to-date on the topic of complementary medicine. The comprehensive information provided in Complementary Medicine Diploma is helpful not just for therapists and professionals but also for any layman interested in the field. What you will learn 1: Introduction to Complementary Medicine 2: Diagnosis in Complementary Medicine 3: Reading the Body 4: Introduction to Traditional Chinese Medicine 5: Understanding Ayurveda 6: Tibetan Medicine 7: Introduction to Japanese Medicine 8: Introduction to Nature Cure 9: Introduction to Acupuncture 10: Introduction to Homeopathy Course Outcomes After completing the course, you will receive a diploma certificate and an academic transcript from Elearn college. Assessment Each unit concludes with a multiple-choice examination. This exercise will help you recall the major aspects covered in the unit and help you ensure that you have not missed anything important in the unit. The results are readily available, which will help you see your mistakes and look at the topic once again. If the result is satisfactory, it is a green light for you to proceed to the next chapter. Accreditation Elearn College is a registered Ed-tech company under the UK Register of Learning( Ref No:10062668). After completing a course, you will be able to download the certificate and the transcript of the course from the website. For the learners who require a hard copy of the certificate and transcript, we will post it for them for an additional charge.
SwiftUI Animations iOS 16 - Animate Anything with SwiftUI
By Packt
Welcome to this course on SwiftUI animations iOS 16. This is a fun-to-code course with multiple hands-on projects geared toward various skill levels. Each project is marked 'Easy', 'Intermediate', or 'Advanced', allowing you to start coding projects according to your skill level and gradually move on to the higher levels when ready.

Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Apache Kafka Complete Developer's Guide
By Packt
Learn core Apache Kafka features along with creating Java, Node.js and Python producers and consumers

CQC Training Course - New Assessment Framework and How To Be 'Outstanding' Bristol
By Green Maze Support
Being Prepared for CQC's New Assessment Framework Course

CQC Training Course - New Assessment Framework and How To Be 'Outstanding' Exeter
By Green Maze Support
Being Prepared for CQC's New Assessment Framework Course

Search By Location
- introduction Courses in London
- introduction Courses in Birmingham
- introduction Courses in Glasgow
- introduction Courses in Liverpool
- introduction Courses in Bristol
- introduction Courses in Manchester
- introduction Courses in Sheffield
- introduction Courses in Leeds
- introduction Courses in Edinburgh
- introduction Courses in Leicester
- introduction Courses in Coventry
- introduction Courses in Bradford
- introduction Courses in Cardiff
- introduction Courses in Belfast
- introduction Courses in Nottingham
