- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
22698 Courses
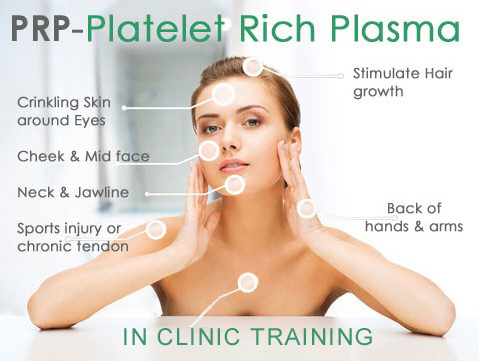
Platelet Rich Plasma Skin & Hair | JOIN IN-CLINIC CLASS
4.9(74)By Harley Elite Academy | Cosmetic Medicine Courses
CLINICAL PRP Sports medicine Traumatology Ophthalmic Burn trauma Wound healing –diabetic foot Skin grafting Dentistry-sinus lift Tooth implants. PRP theory & equipment: Training Online Theory will enable you to understand: Anatomy Vascular Supply, Contraindications Patient consultation Complications Management Post treatment advice Dealing with equipment | Suppliers A certification of training will be provided upon completion of the course. Aesthetic PRP Skin rejuvenation Hair restoration Fat grafting in combination PRP Post laser Acne & Rosacea Acne scar Tissue Volumisation alternative of HA fillers Aesthetic gynaecology /urology. Plathelet Rich Plasma We will cover pertinent information including mechanism of action, safety and efficacy issues, management and treatment of complications, dilution guidelines, and more. Hands on practical session – skin rejuvenation and hair loss Extraction, Preparation and Dosage Management Injection techniques – face, neck and head (hair loss); also the use of cannula Upon successful completion of the course, you will receive a certificate and title of PRP Certified Practitioner. MASTER CLASS PRP & PRF During the course we are providing . Taking blood and how to use a Centrifuge . PRP injecting techniques in face neck and décolletage hands. PRP Microneedling using a DERMAPEN. Combination treatment PRP with Mesotherapy. MECHANISM OF ACTION Platelets + Leucocytes form 3D mesh release of GF Chemo attraction and migration of macrophages and stem cells Stem cells proliferates by mitosis Stem cells undergo differentiation process BENEFIT FROM PRP TREATMENT & THERAPYExperience the advantages of PRP treatment and therapy, utilizing autologous blood with natural growth factors for disease-free and hypoallergenic benefits. Boost wound healing by regulating mitosis, proliferation, and differentiation, enhancing tissue with collagen, elastin, and hyaluronic acid. Benefit from improved tissue oxygenation, nutrition flow, and support for procedures like hair transplants, fat transfers, and skin grafts.PRP works effectively in skin rejuvenation, facial resurfacing, microneedling, and combines well with HA, PDO threads, skin boosters, peeling, or CO2 lasers. It also proves beneficial for hair restoration, showing positive results in various protocols for Androgenic alopecia and age-related hair loss.PRP where works .Skin rejuvenation-facial resurfacing.application-injection alone. Microneedling Combination with HA,Combination with PDO threads,Skin boosters , peeling or CO2 lasers Hair restoration, Multiple protocols with positive results Evidence for improvement of: Androgenic alopecia-male and females, “spot hair lost” Improvement of age related hair loss. You need to be medically qualified as a doctor, dentist, nurse, pharmacist or paramedic with full governing body registration and have completed a Foundation Filler Course and to have administered a number of cases. MASTERCLASS 8 CPD POINTS 1 DAY INTENSIVE COURSE HANDS-ON REAL MODELS
Implementing Good Clinical Laboratory Practice
By Research Quality Association
Course Information Join our comprehensive course, meticulously designed to equip individuals implementing Good Clinical Laboratory Practice (GCLP) within laboratories handling samples from clinical trials. Delve into the current regulatory landscape governing laboratory work supporting clinical trials, referencing key guidelines such as the ICH Guideline for Good Clinical Practice, the Clinical EU Trials Directive, relevant regulations, and leveraging insights from the RQA guidance document on GCLP. Is this course for you? This course is tailored for laboratory managers, analysts, investigators, trial coordinators, monitors, and auditors operating in diverse settings such as pharmaceutical company laboratories, central laboratories, contract research organisations, hospital laboratories, clinics, and investigator sites. This course will give you: Guidance on effectively interpreting and applying GCLP within the broader framework of Good Clinical Practice (GCP) Insight into the seamless integration of GCLP within clinical programmes (GCP) Practical strategies for implementing GCLP in the nuanced environment of clinical research laboratories The chance to update your knowledge with the latest interpretations and guidance on clinical laboratories by the UK Medicines and Healthcare Products Regulatory Agency (MHRA) Access to a seasoned panel of speakers with extensive expertise A unique opportunity to deepen your understanding of GCLP's application across diverse scenarios. Engage in: Lively discussions to foster ideas Problem-solving sessions targeting specific challenges Detailed exploration of specific aspects within the realms of GCP and GCLP. Tutors Tutors will be comprised of (click the photos for biographies): Vanessa Grant -, - Louise Handy Director, Handy Consulting Ltd Tim Stiles Consultant, Qualogy Ltd Programme Please note timings may be subject to alteration. Day 1 08:50 Registration 09:00 Welcome and Introduction 09:20 Good Clinical Practice and the Requirements of Good Clinical Laboratory Practice A review of Good Clinical Practice and its requirements for the laboratory analysis of samples from a trial. The thought processes behind the development of Good Clinical Laboratory Practice, its objective, scope, interpretation and application are explained. 10:00 Safety and Ethical Consideration Informed consent, confidentiality, expedited reporting, blinding and unblinding and serious breaches of the GCP are discussed. 10:40 Break 10:55 Organisation and Personnel Responsibilities within GCP and the Laboratory The responsibilities of key functions that should exist within a clinical laboratory including personnel records of training and competence are discussed. 11:30 Staff Training and Training Records Personnel records of training and competency assessments are discussed. 11:45 Laboratory Facilities, Equipment and Materials Suitable facility design, organisation and operation will be discussed. The calibration, validation and maintenance of equipment used in the conduct of sample analysis are examined, as are the suitability of materials and the identification and labelling of reagents and solutions. 12:30 Lunch 13:15 Workshop 1 - Facilities, Equipment and Responsibilities Some practical problems with regard to the facilities, equipment and responsibilities are explored. 13:45 Workshop 1 - Feedback 14:15 Computer Systems Validation Systems, including computerised systems, used in the analysis, collection and reporting of results should be appropriately tested, operated and controlled. What this means in practice is discussed. 14:45 Trial Protocols, Analytical Plans During this session we examine the purpose, content, control and change of these important documents. 15:30 Break 15:45 Workshop 2 - SOPs, Clinical Protocols, Analytical Plans and Validation The practicalities of managing and documenting the planning phase of analytical work on a trial are explored along with computerised system validation. 16:30 Workshop 2 - Feedback 17:00 Close of Day Day 2 09:00 Conduct of the Work and Quality Control Many of the issues that surround the conduct of sample collection, shipment, storage, analysis and management of Analytical Methods are discussed. This includes the quality control of the assay that may be employed and Quality Control checks. 10:00 Deviation Management The expectations around deviations and CAPA are discussed. 10:15 Workshop 3 - Conduct of the Work and Quality Control Practical work conduct and quality control issues are explored. 10:45 Break 11:00 Workshop 3 - Feedback 11:30 Source Data, Data Integrity, Records and Reports The creation and subsequent management of source data and records, data integrity, are discussed, together with the process of reporting analytical results. 12:10 Workshop 4 - Data, Records and Reports Practical problems with data, records and reports are investigated. 12:45 Lunch 13:30 Workshop 4 - Feedback 14:00 Quality Audit The requirements for and purpose of quality audits are discussed. The difference between quality audit and quality control are explained along with the role of the quality audit staff and their interaction with the analytical project managers, laboratory management and study staff. 14:40 Risk Management How should we assess risk and how can we use the process to assist in evaluation of audit findings. 15:15 Break 15:30 Regulatory Inspection The conduct of regulatory inspections and current expectations of the inspectors. Preparation for inspections and conduct during them will be discussed. 16:00 Panel Session This panel session will address any outstanding issues raised by the delegates. 16:15 Close of Course Extra Information Face-to-face course Course Material Course material will be available in PDF format for delegates attending this course. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course. The material will be emailed in advance of the course and RQA will not be providing any printed copies of the course notes during the training itself. Delegates wishing to have a hard copy of the notes should print these in advance to bring with them. Alternatively delegates are welcome to bring along their own portable devices to view the material during the training sessions. Remote course Course Material This course will be run completely online. You will receive an email with a link to our online system, which will house your licensed course materials and access to the remote event. Please note this course will run in UK timezone. The advantages of this include: Ability for delegates to keep material on a mobile device Ability to review material at any time pre and post course Environmental benefits – less paper being used per course Access to an online course group to enhance networking. You will need a stable internet connection, a microphone and a webcam. CPD Points 14 Points Development Level Develop

Foundation Toxin Course
By Palm Springs Aesthetics
Our foundation anti-wrinkle toxin Course for Medical Professionals is a comprehensive training program designed to equip healthcare practitioners with the necessary skills and knowledge to perform safe and effective anti-wrinkle toxin treatments
The VTCT Level 3 Certificate in Assessing Vocational Achievement (Hairdressing)
4.8(16)By Kleek Academy
Obtain The Assessor's Qualification through our comprehensive teaching and assessing courses at Kleek Training. Develop the skills and knowledge needed to effectively assess learners. Enquire today!

First Aid at Work (3 Days)
By Training Legs First Aid
This 3-day FAA Level 3 Award course offers a variety of first aid situations and teaches how to respond to an emergency. It gives delegates the confidence to deal with any of these situations safely and efficiently.

ServiceTech - Tools and Equipment Training
By Learning for Hire Limited
This course covers road-tow equipment - from legal checks, tyres, lighting through to brake checks, adjustment and repairs. Ideal for tool hire, plant hire. CPA Members HAE Members and IPAF Members for their Fitters, mechanics, technicians, Check and Test, Test and Run, PDI Techs, not forgetting Charities or other organisations who run maintain or sell trailer mounted items nd those that sell, deal in or refurbish equipment.
Reformer L1
By Pilates Performance Ireland
Pilates Reformer Instructor Training Program Modules: Reformer I, The Pilates Reformer is an extraordinarily flexible piece of exercise equipment allowing resistance and support for exercises involving every part of the body. Our program gives you a thorough understanding of how to use the Reformer to develop core and extremity strength, stability, flexibility, coordination and balance.
Introduction to Paper Crafting and Mixed Media - Monday 19.00 - 21.00 or Tuesday 19.00 - 21.00
By Craft4Smiles C.I.C.
A standalone 10 week/session course that will introduce you to the basic materials, tools, equipment and techniques used in paper crafting and mixed media work and to Craft4Smiles C.I.C and our tutors. You will be sent all the materials and equipment needed to complete the course. At the end of each lesson you will have a crafted item to take away to keep or show family and friends. You will be told about the other courses you offer so that you can make an informed choice about further learning.

Foundation Diploma for Higher Education Studies - Level 3 (fast track mode)
4.0(2)By London School Of Business And Research
The objective of the fast track Level 3 Foundation Diploma for Higher Education Studies is to provide learners with a foundation to provide an entry route to UK and international university courses. It is designed to ensure that each learner is equipped with knowledge of study skills, mathematics, computing, society and culture, business and accounting, providing the knowledge and skills to adapt rapidly to change and progress with their learning. Successful completion of the fast track Level 3 Foundation Diploma for Higher Education Studies provides learners with the opportunity to progress to further study or employment. Key Highlights of Foundation Diploma for Higher Education Studies - Level 3 (Fast track mode) Program Duration: 6 Months (Regular duration of 9 months also available) Program Credits: 120 Designed for working Professionals Format: Online No Written Exam. The Assessment is done via Submission of Assignment Tutor Assist available Dedicated Student Success Manager Timely Doubt Resolution Regular Networking Events with Industry Professionals Direct entry into Year 1 of a three-year UK Bachelor's degree LSBR Alumni Status No Cost EMI Option Who is this course for? Open Entry. No formal qualification is required Career path After completion of this course qualification will meet the University standard academic entry requirements. However, each applicant will be subject to individual assessment and other entry requirements which may affect university entry. University of Sunderland, UK Progress to all Undergraduate courses within the Faculty of Business, Law and Tourism Certificates Certificate of Achievement Digital certificate - Included Othm courses: Once you complete the course, you would be receiving a digital copy of your Diploma along with its Transcript which can be downloaded from the awarding body website without any additional charge. You can also order Hard copy certificate by paying a nominal cost directly to the awarding body.

Diploma In Business Enterprise - Level 5 (Fast Track mode)
4.0(2)By London School Of Business And Research
This fast track Diploma In Business Enterprise - Level 5 qualification provides comprehensive coverage of the issues, challenges and disciplines growth organisations or business start-ups face. Learners who want to make a success of their own business venture or to develop their skills in promoting or creating growth in organisations will gain significantly from this qualification. After completing this fast track Diploma In Business Enterprise - Level 5 qualification the inspired student or entrepreneur will gain valuable insights into the characteristics, skills, resources and tools required to drive a growing organisation or business start-up forward. Learners will be required to be proactive and engage with businesses that have growth strategies or start-ups with ambition. Learners will be expected to create ideas and plans that support their personal business goals or those of organisations that they are involved with. Key Highlights of Level 5 Diploma In Business Enterprise qualification are: Program Duration: 6 Months Program Credits: 120 Designed for working Professionals Format: Online No Written Exam. The Assessment is done via Submission of Assignment Tutor Assist available Dedicated Student Success Manager Timely Doubt Resolution Regular Networking Events with Industry Professionals Become eligible to gain direct entry into relevant Undergraduate degree programme. Alumni Status No Cost EMI Option Requirements This fast track Level 5 Diploma in Business Enterprise (Accredited by Qualifi) qualifications has been designed to be accessible without artificial barriers that restrict access and progression. Learners will be expected to hold the following: Learners who have demonstrated some ability and possess Qualifications at Level 4 or an equivalent international qualification OR learners may have a first degree in another discipline and want to develop their own business or shift careers Career path Learners completing the Level 5 Diploma in Business Enterprise can progress to: The Final year of an Undergraduate Degree, or Level 6 Diploma qualifications (click here to view) Directly into employment in an associated profession. Certificates Certificate of Achievement Hard copy certificate - Included Once you complete the course, you would be receiving a Physical hard copy of your Diploma along with its Transcript which we would Courier to your address via DHL or Royal Mail without any additional charge

Search By Location
- EQ Courses in London
- EQ Courses in Birmingham
- EQ Courses in Glasgow
- EQ Courses in Liverpool
- EQ Courses in Bristol
- EQ Courses in Manchester
- EQ Courses in Sheffield
- EQ Courses in Leeds
- EQ Courses in Edinburgh
- EQ Courses in Leicester
- EQ Courses in Coventry
- EQ Courses in Bradford
- EQ Courses in Cardiff
- EQ Courses in Belfast
- EQ Courses in Nottingham