- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
132 Courses
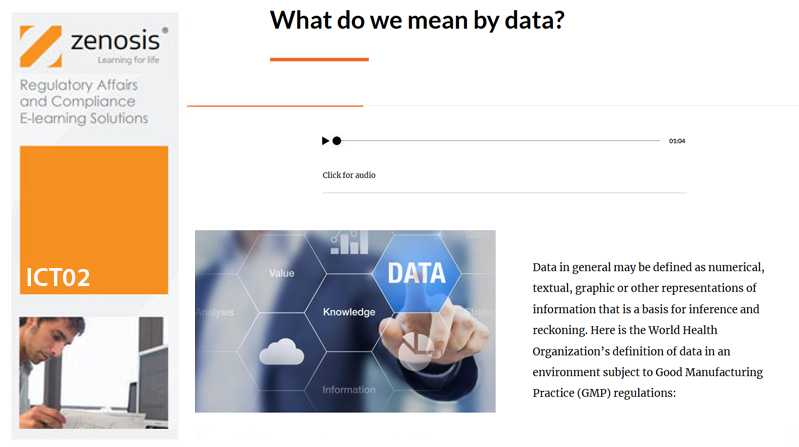
ICT03: Assuring Data Integrity in Clinical Research
By Zenosis
Pharmaceutical, biotechnology and medical device companies and clinical researchers need to assure regulatory authorities of the reliability of the data that they generate during product development and testing – that is, to demonstrate data integrity. Practices that provide assurance of data integrity in clinical research are required by law and/or established as expectations in regulatory guidance. The data are reviewed in regulatory applications or during regulatory inspections of clinical trial sponsor and investigational sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the organizations or individuals concerned. This course explains the requirements and describes principles and practices that should be followed by trial sponsors, investigators and other clinical research personnel to assure regulators of data integrity.

ICT02: Assuring Data Integrity in the Manufacture of Medicinal Products
By Zenosis
Pharmaceutical and biotechnology companies and researchers need to assure regulatory authorities of the reliability of the data that they generate or acquire during product development and manufacturing – that is, to demonstrate data integrity. Data integrity is assessed during regulatory inspections of manufacturing and research sites. Inadequacies of data integrity are frequently reported by inspectors and result in regulatory actions against the companies or individuals concerned. Practices that assure data integrity are required by law and/or expected by regulators in the fields of nonclinical and clinical research, manufacturing and distribution, and pharmacovigilance of medicinal products. This course explains the requirements and describes principles and practices that should be followed to assure regulators and contractual partners of data integrity in the manufacture of medicinal products.
MD01: An Introduction to the Regulation of Medical Devices
By Zenosis
This module provides an introduction to the basics of medical device regulation, especially the requirements that manufacturers must meet in order to market devices in Europe and the USA.

CT12: How to Conduct Clinical Research Under the EU Clinical Trials Regulation
By Zenosis
This course describes the requirements that must be met by, and options available to, the sponsor during the conduct of an authorised clinical trial. It identifies the various interactions with MSCs that occur via the Clinical Trials Information System (CTIS), and it summarises and links to the extensive guidance available from the European Commission and the European Medicines Agency. Its companion course CT11 sets out the European legal and regulatory context for clinical trials and describes how to apply via the CTIS for authorisation to conduct trials. The two courses therefore provide an ideal foundation for understanding and complying with the new law.

PV07: Good Pharmacoepidemiology Practice
By Zenosis
Pharmacoepidemiology is the study of the use and effects of drugs in large numbers of people. It provides a bridge between clinical pharmacology and epidemiology. The increasing demand for real-world evidence of the safety, efficacy and utility of medicinal products has focused greater attention on pharmacoepidemiological research. This module will help those who plan and conduct such research, and analyse and report the findings, to follow good practice.

ESS02: Essentials of Monoclonal Antibodies
By Zenosis
This module will introduce you to monoclonal antibodies, explaining how they work, how they are made, and the many uses to which they are put.

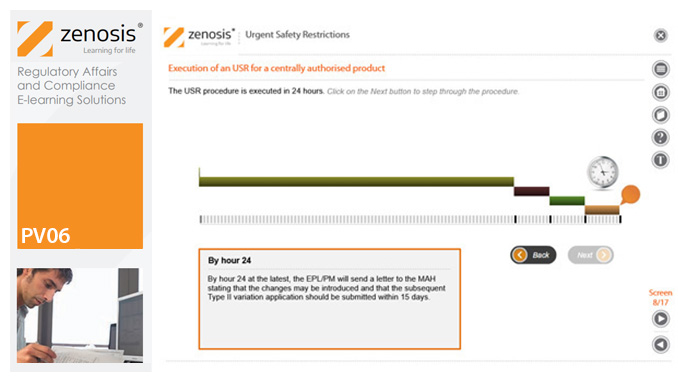
PV06: Urgent Safety Restrictions
By Zenosis
An Urgent Safety Restriction (USR) is a regulatory action taken, in response to a safety signal, to make an interim change to the terms of the marketing authorisation for a medicinal product in Europe. This module describes the principles and procedures for USRs.
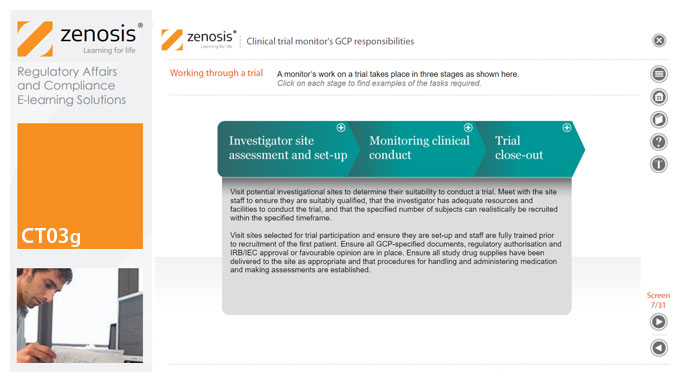
CT03g - Clinical trial monitor’s GCP responsibilities
By Zenosis
A clinical trial monitor acts on behalf of the sponsor to support investigational site personnel, verify the accuracy of data recorded, and ensure that the trial is conducted in compliance with the protocol, GCP and other study specific requirements. He or she acts as the ‘eyes and ears’ of the sponsor at the investigational site and provides the main channel of communication between sponsor and investigator. This short course explores the responsibilities of the monitor and provides insight into key challenges. We discuss assessment of investigators and investigational sites, education and trial initiation, monitoring of clinical conduct, including CRF review and source document verification, and trial close-out. We discuss noncompliance and how to deal with it.
CT04a - Clinical trials in drug development
By Zenosis
New drug development requires major investment in capital, human resources and technical expertise. Strict adherence to regulations on testing and manufacturing standards is also required before a new drug can be marketed. One of the greatest challenges in conducting clinical trials is that of efficiency. As trials become more comprehensive, involving large numbers of participants globally, their duration is prolonged and costs increase. The longer trials last, the shorter is the patent life remaining after market approval and the longer patients must wait for the new product. This short course covers the key components of clinical trials and how these requirements interact with the drug development cycle.

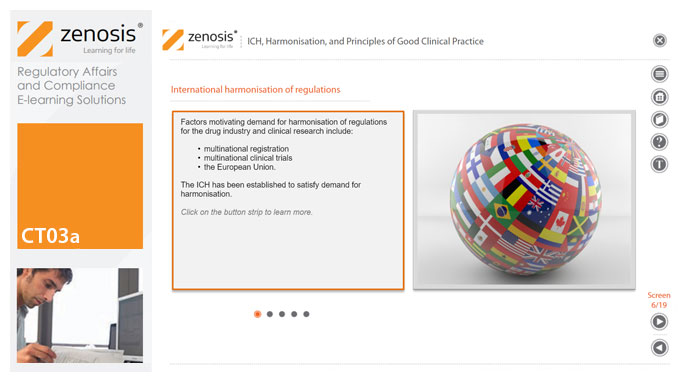
CT03a - ICH, harmonisation, and principles of Good Clinical Practice
By Zenosis
Good Clinical Practice (GCP) is a set of internationally recognised ethical and scientific quality requirements for designing, conducting, recording and reporting clinical trials. Compliance with GCP principles is required by regulatory authorities in many countries for the authorisation of clinical trials and the acceptance of their data in applications for marketing approval. The International Council for Harmonisation's guideline E6, often referred to as ICH GCP, is the international standard specification for Good Clinical Practice. In this short course we describe the ICH’s role in the harmonisation of regulations, introduce its guideline E6, and set out the principles of GCP.
Search By Location
- GMP Courses in London
- GMP Courses in Birmingham
- GMP Courses in Glasgow
- GMP Courses in Liverpool
- GMP Courses in Bristol
- GMP Courses in Manchester
- GMP Courses in Sheffield
- GMP Courses in Leeds
- GMP Courses in Edinburgh
- GMP Courses in Leicester
- GMP Courses in Coventry
- GMP Courses in Bradford
- GMP Courses in Cardiff
- GMP Courses in Belfast
- GMP Courses in Nottingham