- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
Essential OTN training course description An In-depth introduction to the terminology and technology that will comprise tomorrow's Optical Transport Networks. What will you learn Describe the problems with old technologies. Identify the purpose of new technologies. Describe the functionality of the various transmission mediums available Identify OTN features and functionality. Define the issues involved in equipment and application rollout. Essential OTN training course details Who will benefit: Anyone wishing to learn OTN. Prerequisites: SDH foundation or Essential DWDM Duration 2 days Essential OTN training course contents Scope, References Terms and definitions, Abbreviations and Conventions Optical transport network interface structure Multiplexing/mapping principles and bit rates Optical transport module (OTM-n.m, OTM-nr.m, OTM-0.m and OTN 0.mvn) Physical specification of the ONNI Optical channel (OCh) Optical channel transport unit (OTU) Optical channel data unit (ODU) Optical channel payload unit (OPU) OTM overhead signal (OOS) Overhead description and maintenance signals Mapping of client signals and concatenation Mapping ODUk signals into the ODTUjk signal Forward error correction using 16-byte interleaved RS (255,239) codecs ODUk tandem connection monitoring (TCM) overhead OPUk Multiplex Overhead Amendment 2 including: OTN Multiplexing and Mapping, Basic signal structure, ODTU12, ODTU13, ODTU23, OPUk Multiplex Overhead, OPUk Multiplex Structure Identifier (MSI). OPU2 Multiplex Structure Identifier (MSI), OPU3 Multiplex Structure Identifier (MSI), OPUk Payload Structure Identifier Reserved overhead (RES), ODU1 into ODU2 multiplexing, ODU2 into ODU3 multiplexing, ODU1 into ODU3 multiplexing Amendment 3 including: 40 Gbit/s ODU3/OTU3 and 100 Gbit/s ODU4/OTU4, Support of gigabit Ethernet services via ODU0, ODU2e, ODU3 and ODU4, ODU0 and ODUFlex, Multi-lane OTU3 and OTU4 interfaces, Support for InfiniBand Amendment 4 including: OTSn OTN synchronization messaging channel (OSMC) overhead, FC-1600 Amendment 5 Including: ODUk.ts, OTU0LL (OTU0 low latency), OTSiA (optical tributary signal assembly). OTSiG (optical tributary signal group), OTSiG-O (optical tributary signal overhead), CMEP (connection monitoring end- point), CMOH (connection monitoring overhead), MOTU (Multi-OUT), MOTUm (Multi-OTU with management), OTUCn-M (Optical Transport Unit-Cn, with n OxUC overhead instances and 5G tributary slots). SOTU (Single-OUT). SOTUm (Single-OTU with management). Modified bit rates and capacity for OTU1/2/3/4 OTM.nr.m, OTM.n.m, OTM.0.3v4, OTM 0.4v4 Mapping of CBR2G5, CBR10G, CBR10G3 and CBR40G signals into OPUk 64B/66B and 513B block code format PCS lane alignment marker for 40GBASE-R and 100GBASE-R PT=20/PT=21 and AMP/GMP options OTL 4.10 to OTL 4.4 gearbox ODU switching and Line protection Schemes 10 x 10 MSA Overview of current and future coherent and noncoherent technologies 40Gbit and 100Gbit compliant ROADM's Implementers Guide including replacement terms. Differing vendor's equipment and their implementations Individual and group planning exercises: Upgrade a customer STM-64/10G network to a 40G/ OTN network. Upgrade a customer old 16 Wavelength WDM network to be OTN compliant. Implement a new customer 40 wavelength OTU3 OTN compliant MSPP (DWDM) network. Design a cost-effective solution where we can hand over circuits using 'Optical Transport Lanes'.

Online Options
Show all 131GMP01a - GMP – what and why
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. Everyone who works in a processing, quality control, packaging, or warehouse environment for a pharmaceutical or biotechnology company, or one of their contractors, must understand why GMP is important, how it applies to them, and how to comply with it. This short course explains what GMP is and why it is important, and it gives some lessons from history. It introduces the regulations and guidance documents that are the source of GMP rules. Finally, it touches on regulatory inspections and the consequences that can arise from failure to comply with GMP requirements.

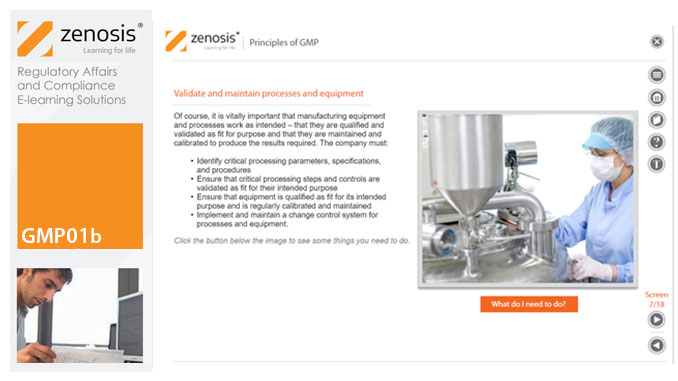
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
GMP01: An Introduction to Good Manufacturing Practice for Medicinal Products
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. The rules may be written into law or set out in guidance documents from regulatory authorities. Regulators will not allow medicinal products to be placed, or to remain, on the market in their country unless the products can be shown to be manufactured in compliance with GMP. To this end, they carry out inspections of manufacturing plants. Companies that persistently commit serious breaches of GMP requirements have suffered huge fines.
Elevate your expertise with our comprehensive Good Manufacturing Practice (GMP) Training Course. Master the essentials of GMP across cosmetics, food, and pharmaceutical industries. Dive into cutting-edge topics like IT applications, contamination control, and maintenance strategies. Join us to foster a culture of quality, compliance, and excellence in manufacturing. Transform your career with industry-relevant knowledge and practical insights. Enroll now for a future where your skills drive the standards of safety and quality.

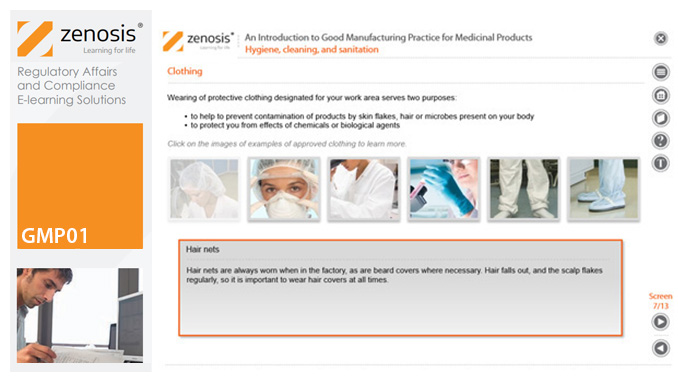
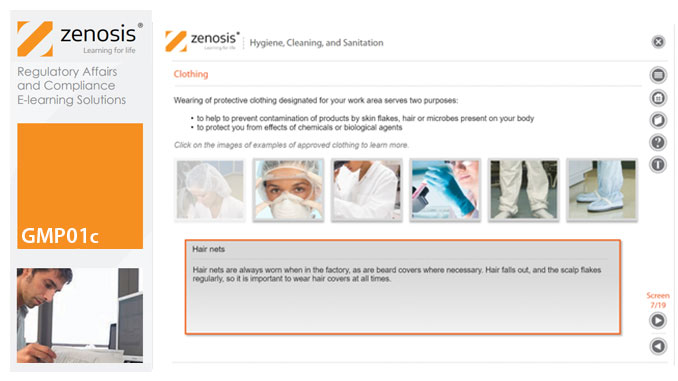
GMP01c - Hygiene, cleaning, and sanitation
By Zenosis
Prevention of contamination is one of the most important goals of GMP. Contamination of product is often difficult to detect, so GMP rules emphasise preventive measures, including: attention to personal health and hygiene, and the wearing of special clothing, by staff; and cleaning and sanitation of premises and equipment. In this short course we set out the basics of GMP requirements in these vital areas.
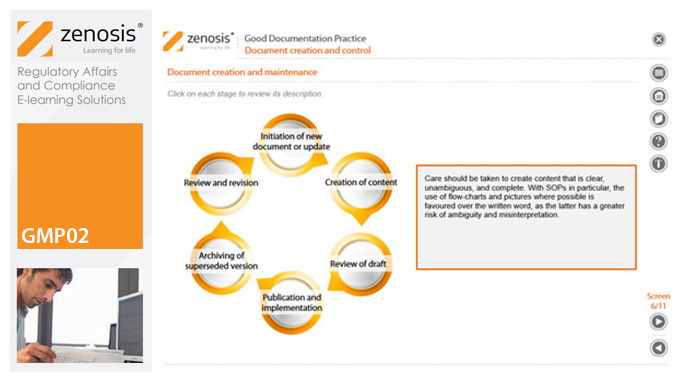
GMP02: Good Documentation Practice
By Zenosis
Good Manufacturing Practice (GMP) for medicinal products relies on documentation. Good Documentation Practice (GDocP) is that part of GMP that applies to the creation, maintenance, use, and retention of documents to provide assurance of the quality of products.
24-Hour Knowledge Knockdown! Prices Reduced Like Never Before Did you know that in the UK, there are over 6,000 manufacturers employing millions of people? But how many ensure their products are consistently safe and effective? This GMP bundle equips you with the knowledge and skills to excel in the demanding world of manufacturing. Are you ready to ensure quality and safety throughout the production process? This comprehensive GMP bundle offers a unique blend of theory and practical knowledge. You'll gain a thorough understanding of Good Manufacturing Practices (GMP), a cornerstone of quality control in various industries. Delve into modern manufacturing techniques, quality management principles, and the roles of Quality Assurance (QA) professionals. Optimise warehouse safety and management, explore logistics and supply chain fundamentals, and even gain insights into procurement practices. Finally, learn valuable SAP Controlling (CO) skills for product costing in S4HANA environments. This Diploma in Good Manufacturing Practice (GMP) at QLS Level 5 course is endorsed by The Quality Licence Scheme and accredited by CPD QS (with 150 CPD points) to make your skill development & career progression more accessible than ever! This Manufacturing & Product Management Bundle Contains 11 of Our Premium Courses for One Discounted Price: Course 01: Good Manufacturing Practices (GMP) Course 02: Modern Manufacturing Course 03: Quality Management Course 04: Quality Assurance (QA) Manager Diploma Course 05: Warehouse Safety Course 06: Warehouse Management Course 07: Logistic Management Course 08: Supply Chain Management Course 09: Purchasing & Procurement Course 10: SAP Controlling (CO) - Product Costing S4HANA Course 11: Operations Management Whether you're new to manufacturing or looking to advance your career, this GMP bundle empowers you to contribute to safe and high-quality production processes. Enrol today and take a significant step towards a rewarding career in the manufacturing industry. Learning Outcomes of Good Manufacturing Practice (GMP) Master Good Manufacturing Practices (GMP) and quality assurance principles. Understand modern manufacturing techniques and quality management methodologies. Implement effective warehouse management and logistics operations. Ensure regulatory compliance and adhere to industry standards. Develop expertise in supply chain management and procurement. Gain insights into SAP product costing and cost control. Why Choose Us? Get a Free CPD Accredited Certificate upon completion of Good Manufacturing Practice (GMP) Get a free student ID card with Good Manufacturing Practice (GMP) Training program (£10 postal charge will be applicable for international delivery) The Good Manufacturing Practice (GMP) is affordable and simple to understand This course is entirely online, interactive lesson with voiceover audio Get Lifetime access to the Good Manufacturing Practice (GMP) course materials The Good Manufacturing Practice (GMP) comes with 24/7 tutor support Start your learning journey straightaway! *** Course Curriculum *** Course 01: Good Manufacturing Practices (GMP) Module 01: Basic Concept of GMP and Safety Regulations Module 02: Good and Bad Manufacturing Practice Module 03: Preventing and Controlling Contamination Module 04: Maintenance and Hygiene Module 05: GMP for Pharmaceutical Industry Module 06: GMP for Food Industry Module 07: GMP for Cosmetics Industry Module 08: IT Applications on GMP Course 02: Modern Manufacturing Section 01: Introduction Section 02: Electric Discharge Machining Section 03: Electrochemical Machining Section 04: Abrasive Jet Machining Section 05: Ultrasonic Machining Section 06: Laser Beam Machining Section 07: Plasma Arc Machining Section 08: Electron Beam Machining Section 09: The Finish Line Course 03: Quality Management Module 01: Introduction To Quality Management Module 02: Total Quality Management Module 03: Quality Measurement And Improvement Module 04: Quality Control Module 05: Understanding Customer Expectations And Needs Module 06: Six Sigma Module 07: Supply Chain Management Module 08: Quality Audits =========>>>>> And 8 More Courses <<<<<========= How will I get my Certificate? After successfully completing the course, you will be able to order your Certificates as proof of your achievement. PDF Certificate: Free (Previously it was £12.99*11 = £143) CPD Hard Copy Certificate: £29.99 CPD 260 CPD hours / points Accredited by CPD Quality Standards Who is this course for? Anyone interested in learning more about the topic is advised to take this bundle. This bundle is ideal for: Quality Professionals Warehouse Personnel Logistics Managers Procurement Specialists Finance Professionals Requirements You will not need any prior background or expertise to enrol in this bundle. Career path After completing this bundle, you are to start your career or begin the next phase of your career. Quality Control Inspector Quality Assurance Manager Warehouse Manager Supply Chain Coordinator Purchasing Agent Cost Analyst Certificates CPD Accredited Digital Certificate Digital certificate - Included Upon passing the Course, you need to order a Digital Certificate for each of the courses inside this bundle as proof of your new skills that are accredited by CPD QS for Free. CPD Accredited Hard Copy Certificate Hard copy certificate - £29.99 Please note that International students have to pay an additional £10 as a shipment fee. Diploma in Good Manufacturing Practice (GMP) at QLS Level 5 Hard copy certificate - £119 Please note that International students have to pay an additional £10 as a shipment fee.

GMP04: Good Manufacturing Practice for the Warehouse
By Zenosis
The warehouse plays a crucial role in a medicinal products factory. This module explains the requirements of Good Manufacturing Practice (GMP) for the warehouse, and how to comply with them.

Overview This comprehensive course on Good Manufacturing Practice (GMP) will deepen your understanding on this topic. After successful completion of this course you can acquire the required skills in this sector. This Good Manufacturing Practice (GMP) comes with accredited certification from CPD, which will enhance your CV and make you worthy in the job market. So enrol in this course today to fast track your career ladder. How will I get my certificate? You may have to take a quiz or a written test online during or after the course. After successfully completing the course, you will be eligible for the certificate. Who is This course for? There is no experience or previous qualifications required for enrolment on this Good Manufacturing Practice (GMP). It is available to all students, of all academic backgrounds. Requirements Our Good Manufacturing Practice (GMP) is fully compatible with PC's, Mac's, Laptop, Tablet and Smartphone devices. This course has been designed to be fully compatible with tablets and smartphones so you can access your course on Wi-Fi, 3G or 4G. There is no time limit for completing this course, it can be studied in your own time at your own pace. Career Path Learning this new skill will help you to advance in your career. It will diversify your job options and help you develop new techniques to keep up with the fast-changing world. This skillset will help you to- Open doors of opportunities Increase your adaptability Keep you relevant Boost confidence And much more! Course Curriculum 9 sections • 9 lectures • 05:38:00 total length •Basic Concept of GMP and Safety Regulations: 00:41:00 •Good and Bad Manufacturing Practice: 00:44:00 •Preventing and Controlling Contamination: 00:37:00 •Maintenance and Hygiene: 00:41:00 •GMP for Pharmaceutical Industry: 00:56:00 •GMP for Food Industry: 00:34:00 •GMP for Cosmetics Industry: 00:54:00 •IT Applications on GMP: 00:31:00 •Assignment - Good Manufacturing Practice (GMP): 00:00:00

Level 5 Diploma Good Manufacturing Practice (GMP) - QLS Endorsed
By Kingston Open College
QLS Endorsed + CPD QS Accredited - Dual Certification | Instant Access | 24/7 Tutor Support | All-Inclusive Cost

Search By Location
- GMP Courses in London
- GMP Courses in Birmingham
- GMP Courses in Glasgow
- GMP Courses in Liverpool
- GMP Courses in Bristol
- GMP Courses in Manchester
- GMP Courses in Sheffield
- GMP Courses in Leeds
- GMP Courses in Edinburgh
- GMP Courses in Leicester
- GMP Courses in Coventry
- GMP Courses in Bradford
- GMP Courses in Cardiff
- GMP Courses in Belfast
- GMP Courses in Nottingham