- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
11307 Courses
CTS FLEX Training (One to One) "FREE" 1 MONTH
By Cts Workout, Sl
CTS FLEX (one-on-one) training is designed to help you TRAINING and improve HEALTH with the CTS WORKOUT Training System anywhere. If you're ready to push your limits and see great results, sign up today! For 3 months you will have FREE ONLINE training with no obligation. Then if you want to continue, just let us know.

Duty of Care
By Prima Cura Training
The duty of care is a legal requirement and comes with the job role for any Care worker. It is part of the code of conduct for healthcare support workers and adult social care workers in England and applies as soon as someone receives treatment or care. Employees also have a duty of care to other workers.

Professional Certificate Course in Introduction to Scheduling In Supply Chain Operations in London 2024
4.9(261)By Metropolitan School of Business & Management UK
This Professional Certificate Course in Introduction to Scheduling in Supply Chain Operations covers the fundamentals of operation scheduling, its objectives, functions, and types. It also covers different approaches to scheduling, load work centers, and sequencing methods. The course introduces performance measures and ways to minimize scheduling difficulties. Additionally, the course focuses on the Theory of Constraints, including its principles, metrics, and scheduling multiple resources. Students will learn about time-cost trade-offs and scheduling services. By the end of this course, students will have a comprehensive understanding of scheduling and optimization techniques in the supply chain. This Professional Certificate Course provides a comprehensive understanding of scheduling and optimization techniques in the supply chain operations. The course covers the theory of constraints, load work centers, sequencing methods, and approaches to scheduling. After the successful completion of the course, you will be able to learn about the following, Operation Scheduling, its Objectives, functions, and types. Approaches to scheduling and Load Work Centres, along with concept and method of sequencing, performance measures, and minimizing scheduling difficulties. Theory of Constraints which includes principles, metrics used, Scheduling Services, Scheduling Multiple Resources, and Time-Cost Trade-Offs. This Professional Certificate Course in Introduction to Scheduling in Supply Chain Operations covers the fundamentals of operation scheduling, its objectives, functions, and types. It also covers different approaches to scheduling, load work centers, and sequencing methods. The course introduces performance measures and ways to minimize scheduling difficulties. Additionally, the course focuses on the Theory of Constraints, including its principles, metrics, and scheduling multiple resources. Students will learn about time-cost trade-offs and scheduling services. By the end of this course, students will have a comprehensive understanding of scheduling and optimization techniques in the supply chain. VIDEO - Course Structure and Assessment Guidelines Watch this video to gain further insight. Navigating the MSBM Study Portal Watch this video to gain further insight. Interacting with Lectures/Learning Components Watch this video to gain further insight. Introduction to Scheduling In Supply Chain Operations Self-paced pre-recorded learning content on this topic. Introduction to Scheduling In Supply Chain Operations Put your knowledge to the test with this quiz. Read each question carefully and choose the response that you feel is correct. All MSBM courses are accredited by the relevant partners and awarding bodies. Please refer to MSBM accreditation in about us for more details. There are no strict entry requirements for this course. Work experience will be added advantage to understanding the content of the course. The certificate is designed to enhance the learner's knowledge in the field. This certificate is for everyone eager to know more and get updated on current ideas in their respective field. We recommend this certificate for the following audience Supply chain professionals Operations managers Logistics professionals Production managers Inventory managers Procurement managers Anyone interested in supply chain scheduling and optimization Average Completion Time 2 Weeks Accreditation 3 CPD Hours Level Advanced Start Time Anytime 100% Online Study online with ease. Unlimited Access 24/7 unlimited access with pre-recorded lectures. Low Fees Our fees are low and easy to pay online.

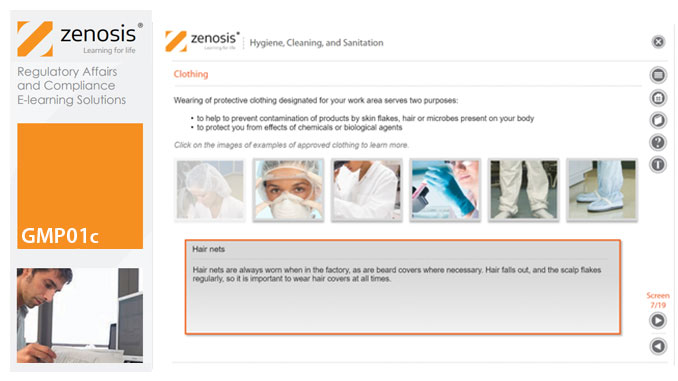
GMP01c - Hygiene, cleaning, and sanitation
By Zenosis
Prevention of contamination is one of the most important goals of GMP. Contamination of product is often difficult to detect, so GMP rules emphasise preventive measures, including: attention to personal health and hygiene, and the wearing of special clothing, by staff; and cleaning and sanitation of premises and equipment. In this short course we set out the basics of GMP requirements in these vital areas.
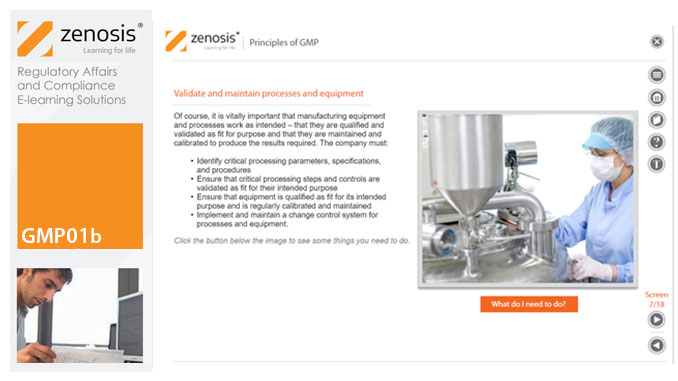
GMP01b - Principles of GMP
By Zenosis
In this short course we present an overview of the main principles of GMP, and we outline some things that manufacturing personnel need to do to comply with requirements. We identify the principal goals of GMP as: prevention of contamination; prevention of mix-ups; scrupulous documentation; validation and maintenance of processes and equipment; quality assurance by an independent unit; and training. We place GMP in the context of a company’s quality management system.
GMP01a - GMP – what and why
By Zenosis
Good Manufacturing Practice (GMP) is a set of rules for medicines manufacturers to follow so that their products are safe, effective, and of good quality. Everyone who works in a processing, quality control, packaging, or warehouse environment for a pharmaceutical or biotechnology company, or one of their contractors, must understand why GMP is important, how it applies to them, and how to comply with it. This short course explains what GMP is and why it is important, and it gives some lessons from history. It introduces the regulations and guidance documents that are the source of GMP rules. Finally, it touches on regulatory inspections and the consequences that can arise from failure to comply with GMP requirements.

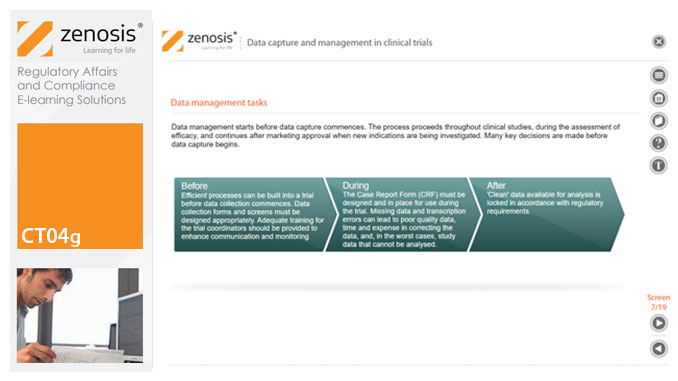
CT04g - Data capture and management in clinical trials
By Zenosis
Capture and management of clinical trial data is a challenge. The industry is under pressure to obtain and analyse such data more quickly, while maintaining data integrity, so that products can be brought to market sooner. Effective planning and adequate resources can ensure clinical trials yield high quality data within strict timelines and budget requirements, at the same time satisfying regulatory standards. This short course describes the purpose of data capture and explores efficiencies in data management as part of the evolving regulatory landscape.
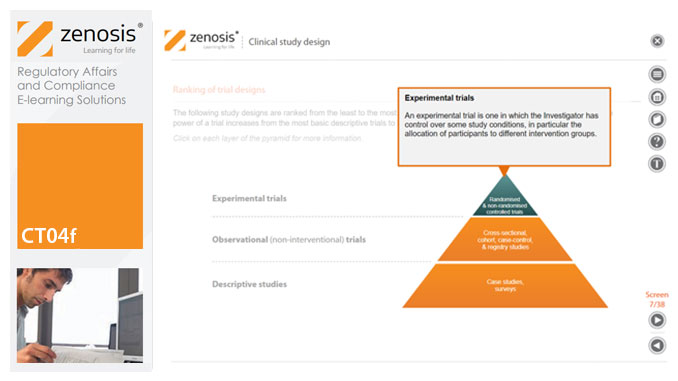
CT04f - Clinical study design
By Zenosis
Clinical trial design establishes the framework upon which the clinical trial process will be conducted, and sets the objectives of the trial. The application for marketing approval, submitted to the regulatory authorities, will provide clinical data reflecting the trial design. Since trial design impacts the whole drug development process and lifecycle, particular care and due diligence is essential. This short course provides an overview of the main types of study design.
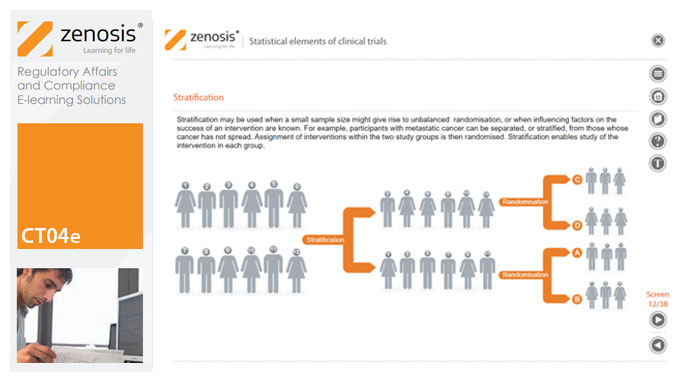
CT04e - Statistical elements of clinical trials
By Zenosis
Analytical statistical elements are essential concepts in the design of clinical trials. This analysis helps us to understand whether a conclusion from a study of a sample of the target population applies generally to that population as a whole. In particular, it helps us to answer the question: Did the treatment effect in the given study occur just by chance? The statistical elements of a well-controlled study minimise the chances of drawing the wrong conclusions, by providing clear thresholds for such errors. The basic statistical elements of a clinical trial include eligibility criteria, randomisation, sample size, power, and blinding, and these are discussed in this short course.
CT04d - Clinical trial endpoints
By Zenosis
In clinical trials, endpoints are measurements to evaluate the results of a new treatment, at an individual patient level. The study data can be extrapolated to patient populations on the basis of clinical similarities to patients participating in the trial. When clinical trial data have been obtained, focus is on the trial endpoints; more specifically, the focus is on whether the trial met or failed the primary endpoint specified before the trial started. The purpose and various types of endpoints are discussed in this short course.
Search By Location
- Safety Courses in London
- Safety Courses in Birmingham
- Safety Courses in Glasgow
- Safety Courses in Liverpool
- Safety Courses in Bristol
- Safety Courses in Manchester
- Safety Courses in Sheffield
- Safety Courses in Leeds
- Safety Courses in Edinburgh
- Safety Courses in Leicester
- Safety Courses in Coventry
- Safety Courses in Bradford
- Safety Courses in Cardiff
- Safety Courses in Belfast
- Safety Courses in Nottingham