- Professional Development
- Medicine & Nursing
- Arts & Crafts
- Health & Wellbeing
- Personal Development
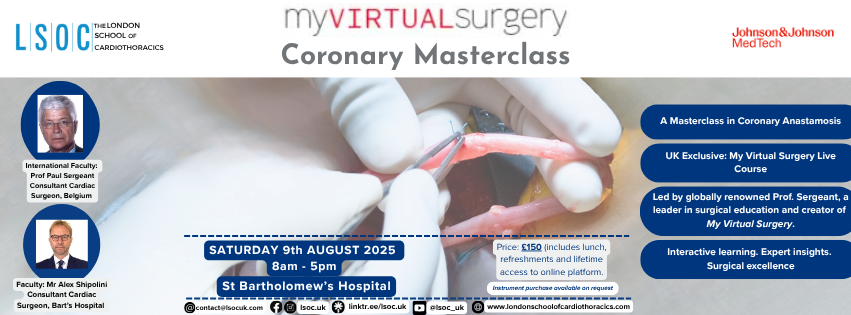
My Virtual Surgery - Coronary Masterclass A Dry-Lab Masterclass in Coronary Anastomosis A NEW LSOC Course! Led by My Virtual Surgery Founder, and Internationally - renowned coronary surgeon, Professor Paul Sergeant, and expert senior Cardiac Surgeon, Mr Alex Shipolini. This course provides a comprehensive masterclass in coronary anastomosis, featuring innovative anastomotic techniques, specialised surgical ergonomics, and expert guidance. PLEASE REVIEW THE IMPORTANT INFORMATION BELOW BEFORE PURCHASING YOUR TICKET (including regarding pre-course learning). When: Saturday, 9th August 2025. Where: St.Bartholomew's Hospital, London. Required Equipment: Needle holder and forceps for pre-course learning (see below). Ticket Types: Course Only This will provide access to the elements detailed below. This does NOT include the costs of instruments. Please purchase this ticket if you have access to a set of needle holders and thin-tipped forceps at home (ideally a Castroviejo needle holder and Geralds/Ring tip forceps). Course + Instruments This will provide course access + procurement and shipping of a Castroviejo needle holder and Atraumatic/Geralds/Ring tip forceps, for you to keep. Please purchase this ticket if you would also like to purchase instruments. Cost includes the course cost, instrument costs, inc. shipping, processing and handling fees. All Tickets include: Access to pre-course training with the MyVirtualSurgery Programme. All day face-to-face seminar on coronary artery surgery from coronary experts. Lifetime access to MyVirtualAnastamosis - the MVS Coronary Anastomosis platform, including direct feedback at home from Prof Sergeant, and ongoing virtual learning. Additionally included in the Course and Instrument Ticket: Procurement of Castroviejo Needle Holder and Atraumatic Forceps to support home training. Sales Open: 22nd June, 7pm Please note: Mandatory Pre-Course Learning Pre-Requisites Pre - Course Learning Deadline All participants must complete Mandatory Pre-Course Learning before the course begins. To ensure we can prepare adequately for the course, including confirming the number of attendees who have completed the pre-course training, a fixed deadline of Monday 28th July will be set for all participants to finish the pre-course training. Not completing the pre-course learning by Monday, 28th July, will lead to forfeiting your place in the course, and no refunds will be issued. Pre-Course Learning Instruments This pre-course learning requires access to a needle holder and thin-tipped forceps. We strongly recommend using a Castroviejo needle holder along with either Geralds/Ring Tips/Atraumatic forceps, as this platform is designed for these instruments, and alternatives may not provide as comprehensive training. Furthermore, these are essential instruments for cardiac surgery and will be used during the course. These instruments will also be required for the remainder of the steps on the platform. If you cannot independently acquire a needle holder and forceps, LSOC can purchase a Castroviejo needle holder and a pair of forceps on your behalf and send them directly to you. In this case, please make sure to purchase a Course + Instruments Ticket, to include the cost of these instruments. We look forward to you joining us on our newest course soon!
Change Management - Change Matters 1 Day Training in London
By Mangates
Change Management - Change Matters 1 Day Training in London

Change Management - Change Matters 1 Day Training in Bromley
By Mangates
Change Management - Change Matters 1 Day Training in Bromley

Change Management - Change Matters 1 Day Training in Heathrow
By Mangates
Change Management - Change Matters 1 Day Training in Heathrow

Essential Management Skills 1 Day Workshop in London
By Mangates
Essential Management Skills 1 Day Workshop in London

Managing a Virtual Team 1 Day Training in London
By Mangates
Managing a Virtual Team Skills 1 Day Training in London

Search By Location
- TEC Courses in London
- TEC Courses in Birmingham
- TEC Courses in Glasgow
- TEC Courses in Liverpool
- TEC Courses in Bristol
- TEC Courses in Manchester
- TEC Courses in Sheffield
- TEC Courses in Leeds
- TEC Courses in Edinburgh
- TEC Courses in Leicester
- TEC Courses in Coventry
- TEC Courses in Bradford
- TEC Courses in Cardiff
- TEC Courses in Belfast
- TEC Courses in Nottingham

